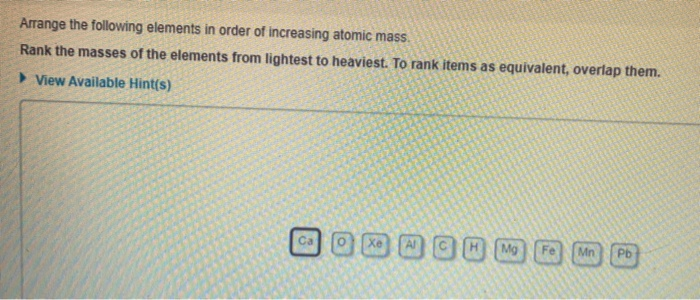
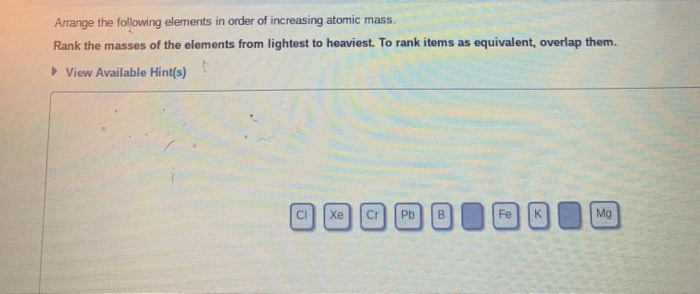
Arrange The Following Elements In Order Of Increasing Atomic Mass.
Ever found yourself staring at a jumble of letters and numbers, wondering what it all means? Well, get ready to unlock a little piece of the universe's puzzle! We're about to dive into the fascinating world of atomic mass and learn how to put some of its fundamental building blocks in order. It's not just about memorizing facts; it's about understanding the very essence of what makes up everything around us, from the air we breathe to the phones in our pockets. Think of it like a cosmic scavenger hunt, where the prize is a deeper appreciation for the intricate dance of elements that form our reality.
So, why is this topic worth your time? Because understanding atomic mass is like having a secret decoder ring for the periodic table. It's the key to understanding how different elements behave, why some react explosively while others remain stubbornly inert, and how they combine to create the incredible diversity of materials we encounter daily. Plus, it's a fantastic way to boost your brainpower! Putting things in order, understanding relationships, and grasping fundamental concepts are all excellent mental exercises that can sharpen your focus and improve your problem-solving skills. It's a fun challenge that pays off with a clearer picture of the world.
The Cosmic Ladder: What is Atomic Mass and Why Order Matters
At its core, atomic mass is essentially the "weight" of an atom. Imagine each atom as a tiny LEGO brick. Some bricks are lighter, made of fewer pieces, while others are heavier, built with more components. Atomic mass tells us how many protons and neutrons are packed inside an atom's nucleus. The more protons and neutrons an atom has, the heavier it is. It's a fundamental property that dictates so much about an element's behavior.
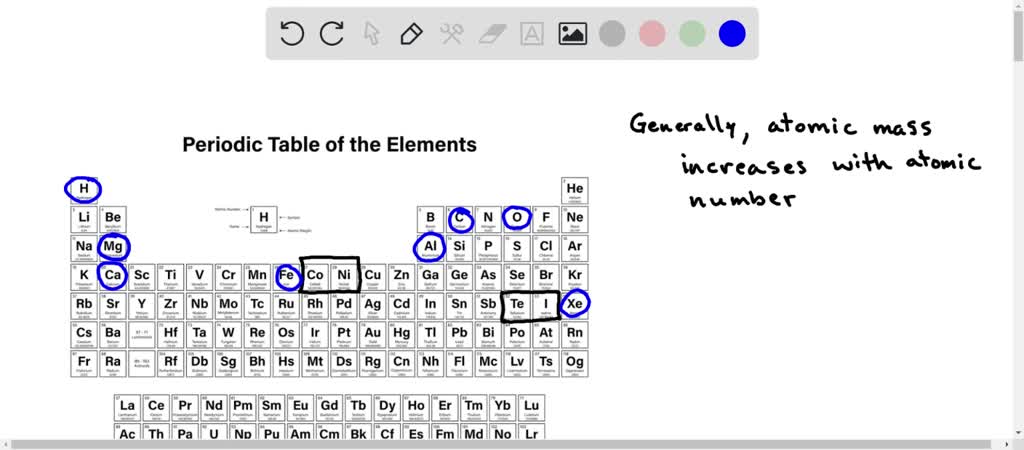
But why bother arranging them? Think about it: if you have a pile of objects, wouldn't it be easier to understand them if they were sorted by size, color, or weight? The same applies to elements. Arranging them by increasing atomic mass creates a structured system, a cosmic ladder, if you will, that reveals patterns and relationships. This order is the very foundation of the periodic table, a masterpiece of scientific organization that scientists have used for centuries to predict and understand the properties of elements.
The benefits of this seemingly simple act of ordering are immense. For scientists, it’s like having a roadmap to the elements, allowing them to predict chemical reactions, design new materials, and understand complex processes. For us, as curious minds, it’s a gateway to appreciating the elegant simplicity and profound complexity of nature. It helps us understand why water (H₂O) is so different from salt (NaCl), even though they’re both made from common elements. It’s all about the underlying masses and how they interact.

Let's take a peek at a few elements that, when placed in order of increasing atomic mass, reveal a fascinating trend. We'll be looking at:
- Hydrogen (H)
- Helium (He)
- Lithium (Li)
- Beryllium (Be)
This might seem like a small group, but the principles at play here are universal. By understanding the atomic mass of these basic building blocks, we begin to grasp the fundamental forces that govern the universe. It's about starting with the simplest and gradually building up our understanding.
Consider Hydrogen (H). It’s the lightest element, the simplest atom with just one proton. Its atomic mass is incredibly small, making it the starting point on our cosmic ladder. Then comes Helium (He), the second lightest, with two protons and two neutrons. It's a bit heavier, a step up on our ladder. Following Helium, we encounter Lithium (Li), a more energetic element with three protons and four neutrons, making it noticeably heavier than Helium.
Finally, we reach Beryllium (Be). With four protons and five neutrons, Beryllium sits a comfortable distance above Lithium in terms of atomic mass. Each step up this ladder represents an increase in the number of fundamental particles within the atom, leading to distinct properties and behaviors. It’s this orderly progression that allows scientists to predict how these elements will interact. For instance, knowing the relative masses helps chemists understand how much of one element is needed to react with another.
The beauty of the periodic table lies in its underlying order. By arranging elements based on properties like atomic mass, we unlock a universe of predictable patterns and profound insights.
So, the next time you hear about atoms or elements, remember this simple concept of ordering by mass. It’s not just a scientific exercise; it’s a way of appreciating the fundamental architecture of our universe, one tiny, ordered step at a time. It's the foundation upon which all chemistry and physics are built, and understanding it is like gaining a new superpower for interpreting the world around you.
