Arrange The Following Elements In Order Of Decreasing Atomic Size
Hey there, science buddies! Ever looked at the periodic table and thought, "Whoa, that's a lot of stuff. But how do these atoms, like, stack up against each other in size?" Today, we're going to dive into a super fun topic: figuring out the atomic size of a bunch of elements and arranging them from biggest to smallest. Think of it like a friendly competition where we're judging who's the most "spacious" atom on the block! No need to break out your lab coats; we're keeping this light and breezy, like a gentle breeze through an open window.
So, the elements we're going to play with today are: Neon (Ne), Potassium (K), Sulfur (S), and Argon (Ar). They're all part of the amazing periodic table, which is basically a cheat sheet for all the elements. If you've ever seen it, it looks a bit like a crazy mosaic, right? But it's actually incredibly organized. And that organization is key to understanding why some atoms are bigger than others.
Before we start ranking, let's chat about what "atomic size" even means. It's not like we can whip out a ruler and measure an atom, although that would be pretty neat! Instead, scientists use something called the atomic radius. Think of it as the distance from the center of the atom (the nucleus, where all the protons and neutrons hang out) to the outer edge of the electron cloud. It’s like the radius of a tiny, fuzzy ball. The bigger this radius, the bigger the atom.
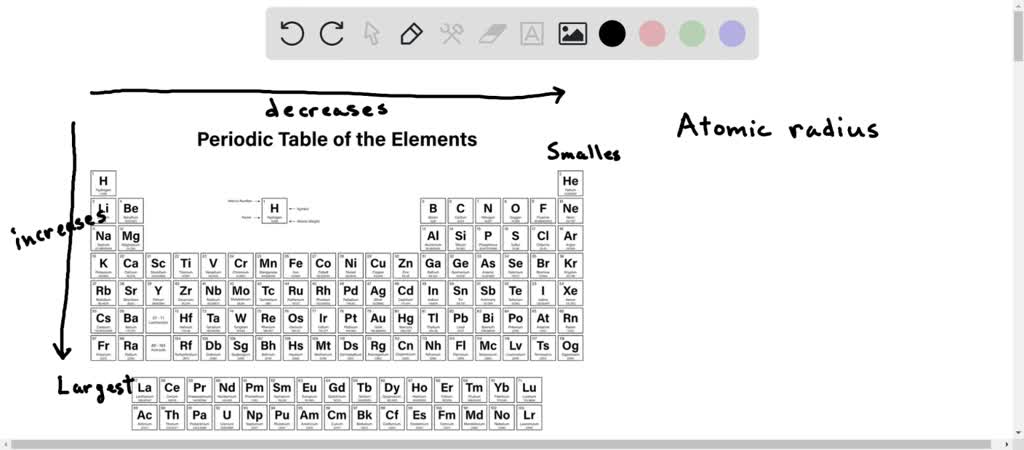
Now, there are a couple of main rules that govern atomic size. It's like the secret handshake of the periodic table. The first big rule is that atomic size generally increases as you go down a group. Groups are the vertical columns on the periodic table. Why? Because as you go down a group, you're adding more electron shells, or energy levels, for the electrons to live in. Each new shell is further away from the nucleus, so the atom just gets bigger and bigger. Imagine adding extra floors to a building; it's going to get taller!
The second big rule is that atomic size generally decreases as you move across a period from left to right. Periods are the horizontal rows on the periodic table. This one's a bit trickier, but it's because as you move across a period, the number of protons in the nucleus increases. This stronger positive charge in the nucleus pulls the electrons in closer, making the atom shrink. It's like a stronger magnet is attracting the electron cloud more powerfully. So, the elements on the left side of a period tend to be bigger than the ones on the right.
Let's put these rules into action with our featured friends: Neon (Ne), Potassium (K), Sulfur (S), and Argon (Ar). First, we need to find them on the periodic table and see where they live.
Potassium (K) is in Group 1, Period 4. It's an alkali metal, and those guys are known for being pretty… generous with their electron shells.
Sulfur (S) is in Group 16, Period 3. It's a nonmetal, and they tend to be a bit more compact.
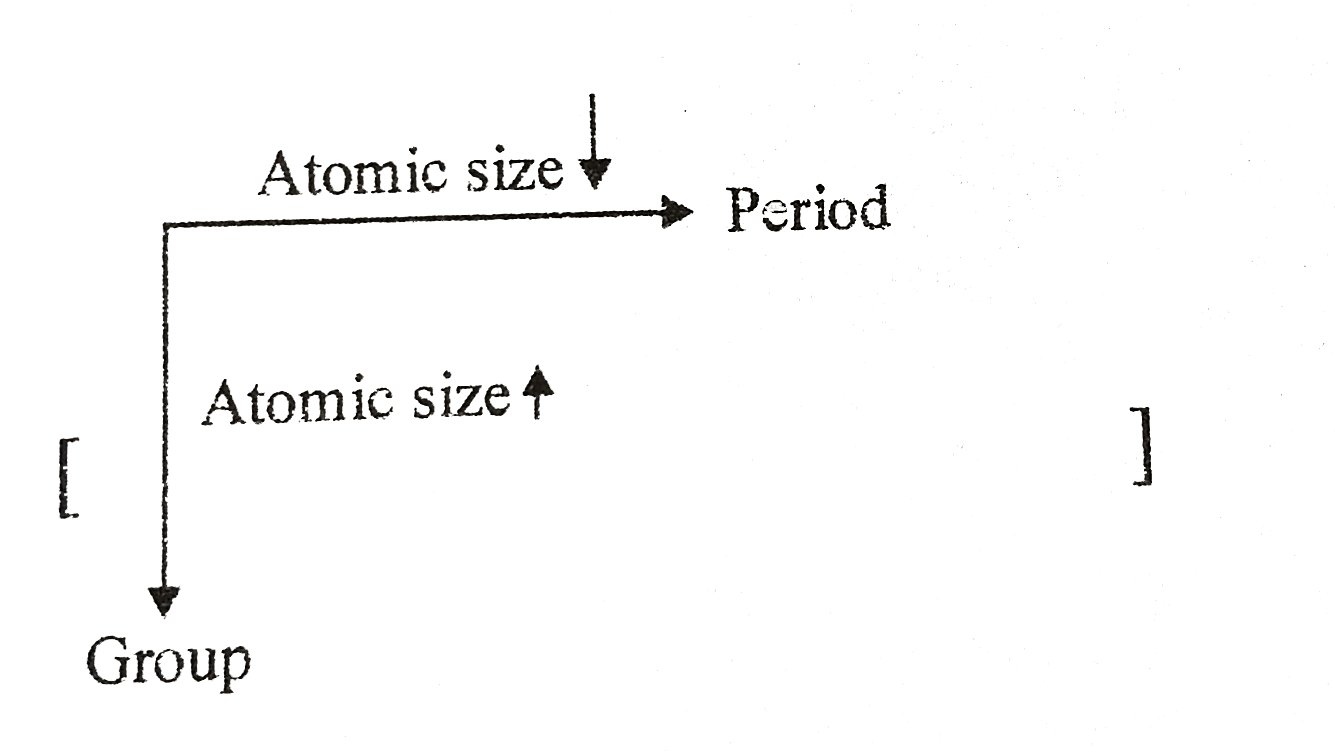
Neon (Ne) is in Group 18, Period 2. It's a noble gas, and noble gases are all about their own little bubble, being super unreactive and, as we'll see, quite small.
Argon (Ar) is also a noble gas, like Neon, but it's in Group 18, Period 3. Aha! This tells us something important already.
Okay, let's break it down element by element, thinking about their positions.
Let's Start with Potassium (K)!
Potassium is in Period 4. Remember our rule about going down groups? Well, even though we're not comparing elements within the same group yet, being in Period 4 means it has electrons in the 4th energy shell. That's a lot of real estate! Generally, elements in higher periods are going to be larger because they have more electron shells. So, Potassium is probably going to be our biggest guy. Think of it as the towering skyscraper of our little element group. It’s got a lot of floors!
Now, let's look at Neon (Ne), Argon (Ar), and Sulfur (S). They are all in different periods and groups, but let's focus on the trends.
Comparing Neon (Ne) and Argon (Ar)
Neon and Argon are both noble gases, which is super cool because they are very stable and don't really like to interact with other elements. They are in the same group (Group 18), but Argon is in Period 3, while Neon is in Period 2. Remember our first rule? Elements get bigger as you go down a group. So, because Argon is below Neon in Group 18, Argon is going to be bigger than Neon. It has an extra electron shell!
So far, we know Potassium is likely the biggest, and Argon is bigger than Neon. We've got this!
Now, Where Does Sulfur (S) Fit In?
Sulfur is in Period 3, Group 16. Now, this is where it gets interesting because we have to compare it to elements in the same period or group. Argon is also in Period 3, but it's in Group 18. Remember our second rule? Elements get smaller as you move across a period from left to right. Argon is way over on the right side of the periodic table (Group 18), and Sulfur is a bit more to the left (Group 16). This means that Sulfur should be larger than Argon.
Think about it: both Sulfur and Argon have electrons in the 3rd energy shell. That's the same number of shells. However, Argon has more protons in its nucleus (18) compared to Sulfur (16). That stronger positive pull from Argon's nucleus is going to draw its electron cloud in tighter, making it smaller than Sulfur. It's like having more people in a room trying to share the same space – things get a bit more crowded and compact!
Putting It All Together: The Big Reveal!
Let's recap what we've figured out:
- Potassium (K) is in Period 4, meaning it has the most electron shells out of our group. This is a huge indicator of size. So, Potassium is definitely the largest.
- Sulfur (S) is in Period 3, Group 16.
- Argon (Ar) is in Period 3, Group 18. Since it's to the right of Sulfur in the same period, Sulfur is larger than Argon.
- Neon (Ne) is in Period 2, Group 18. Since Argon is below Neon in the same group, Argon is larger than Neon.
So, the order from largest to smallest should be:
Potassium (K) > Sulfur (S) > Argon (Ar) > Neon (Ne)
Let's double-check. Potassium is in the 4th period, so it's got the most electron shells. That makes it the biggest. Sulfur and Argon are in the 3rd period. Argon is to the right of Sulfur, so Sulfur is bigger than Argon. Neon and Argon are in the same group, but Neon is above Argon. So, Argon is bigger than Neon. Yep, it all lines up!
It's pretty neat how these simple rules can help us predict the behavior and properties of elements. It's like having a secret decoder ring for the universe of atoms!
So, to summarize our delightful little journey into atomic size, the order of decreasing atomic size for Neon, Potassium, Sulfur, and Argon is:
Potassium (K) is the granddaddy of them all, with its impressive number of electron shells.
Next up is Sulfur (S), a good-sized atom with its electrons in the third shell, but not quite as tightly packed as its noble gas neighbor.
Then comes Argon (Ar), a bit more compact than Sulfur due to the stronger nuclear pull in the same period.
And finally, Neon (Ne) is the smallest, with fewer electron shells and a tighter hold on its electrons.
It’s like a little parade of elements, each with their own unique footprint on the universe. Isn't science just the coolest? You can take a bunch of seemingly random bits of matter and, with a little logic and understanding of trends, you can put them in order. It's like solving a puzzle where the pieces are the building blocks of everything!
So, next time you glance at the periodic table, remember that it's not just a bunch of boxes with letters. It's a beautifully organized map that tells us so much about how these tiny things behave and interact. And understanding atomic size is just one of the many fascinating insights it offers. Keep exploring, keep questioning, and keep that curious spark alive. You’ve totally got this, and the universe of science is yours to discover, one atom at a time!
