Arrange The Following Elements By Increasing Electronegativity.
Hey there, science curious pals! Ever felt like you're just... going through the motions? Well, what if I told you that even something as seemingly "science-y" as arranging elements by their electronegativity could actually add a little sparkle to your everyday life? Yep, you heard me right! It’s not just for lab coats and chalkboards, folks. Let’s dive into the wonderful world of atoms and discover how a bit of electronegativity can be surprisingly… electrifying!
So, what in the name of all that’s chemically cool is electronegativity, you ask? Think of it like this: atoms, the tiny building blocks of everything around us, have little "hands" they use to grab onto electrons. Electronegativity is basically an atom's desire or strength in pulling those electrons closer to itself when it’s bonded with another atom. It’s like a microscopic popularity contest for electrons, and some atoms are just way more popular than others!
Now, we're going to do a little rearranging, a bit of a cosmic sorting ceremony. We're going to take a few of our elemental buddies and line them up, not by size, or color, or even how fancy their names sound, but by how much they want those electrons. Get ready to be amazed as we witness the subtle, yet powerful, dance of electron attraction!
Let’s Get Our Elements in a Row!
Imagine you have a bunch of friends, and you’re all sharing a pizza. Some friends are super enthusiastic, reaching for the biggest slices, while others are a bit more laid back. Electronegativity is that eagerness to snag a slice of electron-pizza!
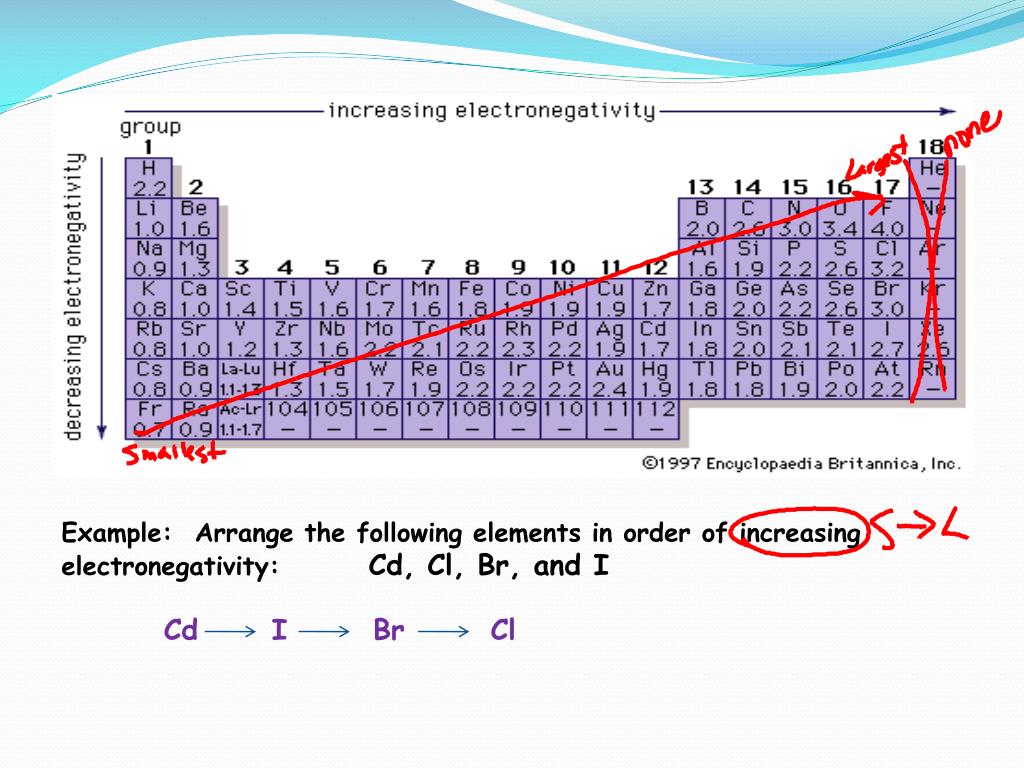
We're going to take these specific elements: Sodium (Na), Aluminum (Al), Phosphorus (P), and Sulfur (S). They’re all in the same row, the third period, of the periodic table. This is important because things get a little predictable when they’re neighbors!
So, how do we arrange them by increasing electronegativity? Think about it like a gentle climb. We start with the element that’s least bothered by electrons and end with the one that’s practically begging for them.

First up, we have Sodium (Na). Sodium is like the chill friend at the pizza party. It’s got plenty of its own electrons and isn’t really looking to hog anyone else’s. It’s happy to share, and frankly, it’s not going to fight hard to pull electrons towards itself. So, Sodium kicks off our increasing electronegativity list. It's at the bottom of the electron-grabbing ladder.
Next on our journey is Aluminum (Al). Aluminum is a bit more interested than Sodium. It’s starting to feel the pull, wanting to be a little closer to those electron-snacks. It’s not a super-grabber yet, but it’s definitely more motivated than Sodium. So, Aluminum takes its spot, just a little higher up the electronegativity scale.
Now we're getting somewhere! Enter Phosphorus (P). Phosphorus is really starting to show some muscle. It's actively looking to attract electrons more strongly than Aluminum. It's like that friend who's getting really excited about the pizza and isn't afraid to reach a little further. Phosphorus is a significant step up in its electron-wanting game!

And finally, the champion of our little electron tug-of-war in this group: Sulfur (S)! Sulfur is very keen on electrons. It’s practically leaning forward, ready to pull them in with all its might. Compared to Sodium, Aluminum, and Phosphorus, Sulfur has the strongest desire to attract electrons. It’s the friend who might just snatch the last pepperoni!
The Grand Reveal!
So, drumroll please! Arranged by increasing electronegativity, our elements look like this:
Sodium (Na) < Aluminum (Al) < Phosphorus (P) < Sulfur (S)
Isn’t that neat? It’s a simple order, but it tells us so much about how these atoms interact. This little list can predict how they'll form bonds and what kind of chemical reactions they’ll be involved in. It’s like knowing your friends' personalities – you can predict who’s going to take the lead on a project or who’s going to be the more reserved one.
Why This Makes Life More Fun (Seriously!)
Okay, so you know the order. How does that make your life more fun? Well, think about it! Understanding these basic principles helps us appreciate the world around us in a whole new light. That water you're drinking? Its properties are a direct result of the electronegativity differences between oxygen and hydrogen!
The vibrant colors of a sunset? The way your phone screen works? The food you eat? All of it, at a fundamental level, is influenced by how atoms play with electrons. When you understand electronegativity, you’re not just memorizing facts; you’re unlocking a secret code to how the universe operates.
It’s like learning a new language – the language of chemistry! Suddenly, everyday objects aren’t just objects; they’re intricate chemical structures governed by fascinating rules. You start noticing patterns, making connections, and feeling a sense of wonder at the complexity and elegance of it all.
Plus, imagine the conversations you could have! "Oh, this dish is so flavorful because the molecules are interacting based on their electronegativity differences!" Okay, maybe that's a little niche, but you get the idea! It adds a layer of appreciation and understanding to everything.
This knowledge empowers you. It’s not about being a genius; it’s about being curious and seeing the magic in the mundane. It’s about realizing that even the smallest, most invisible particles have a fascinating story to tell.
So, the next time you look at something, anything at all, remember that it’s all thanks to the intricate dance of atoms and their electron desires. Embrace that curiosity! Let it lead you down rabbit holes of discovery. You never know what amazing things you’ll learn, or how much more vibrant your world will become.
Keep exploring, keep questioning, and keep finding the fun in science! The universe is brimming with wonders, and understanding even a little bit about how it works can be the most inspiring adventure of all. Go forth and be wonderfully, scientifically curious!
