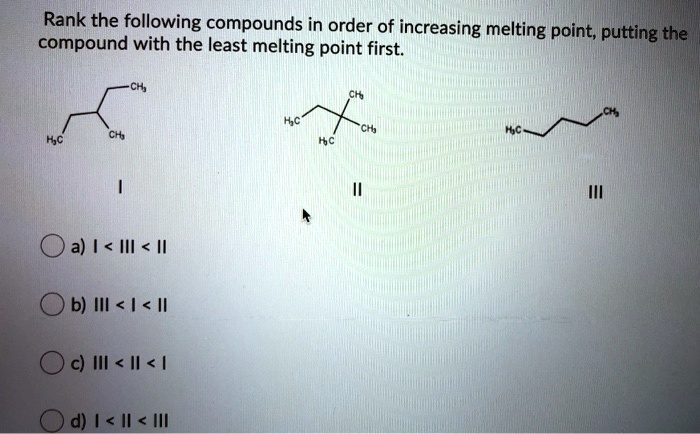
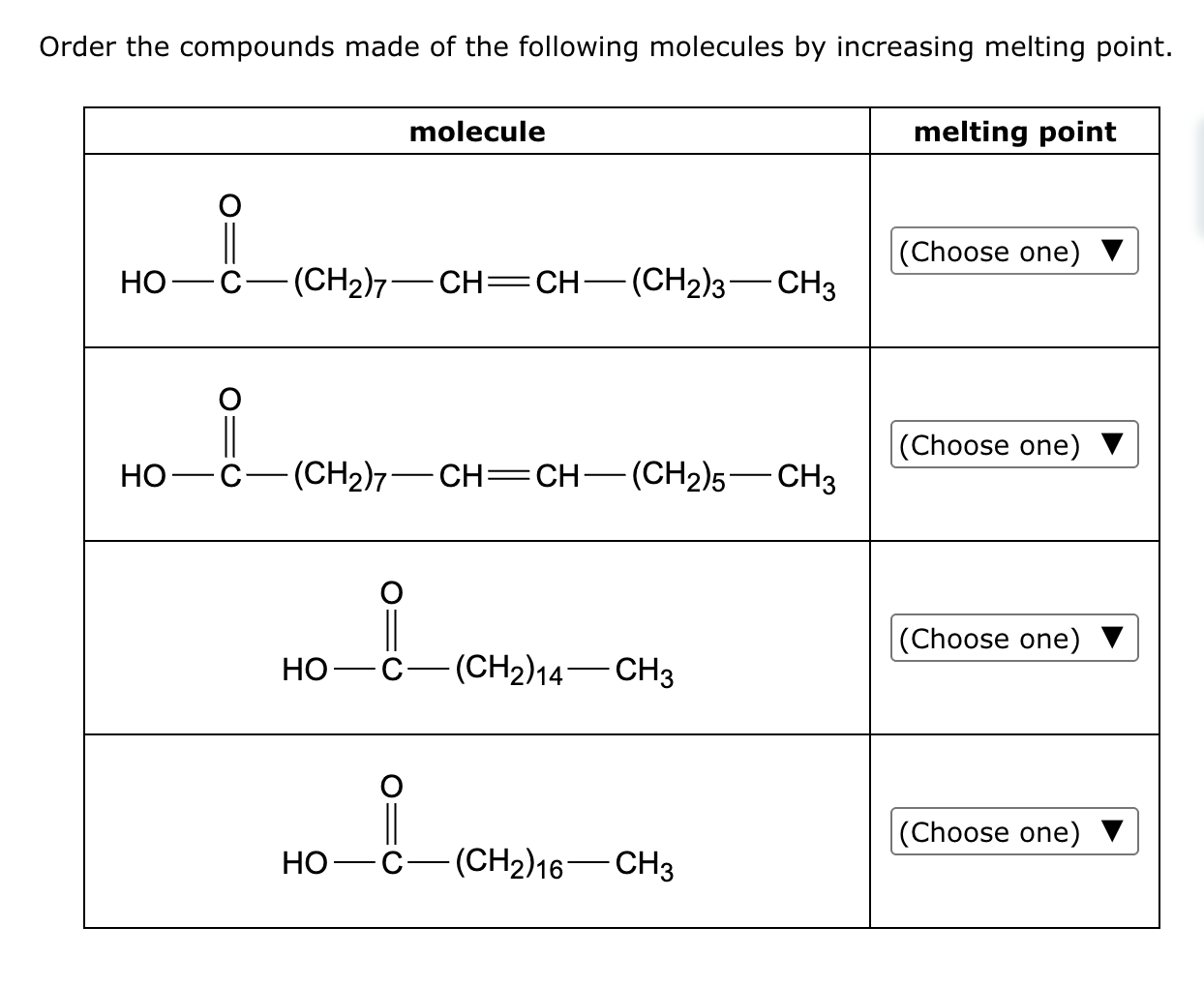
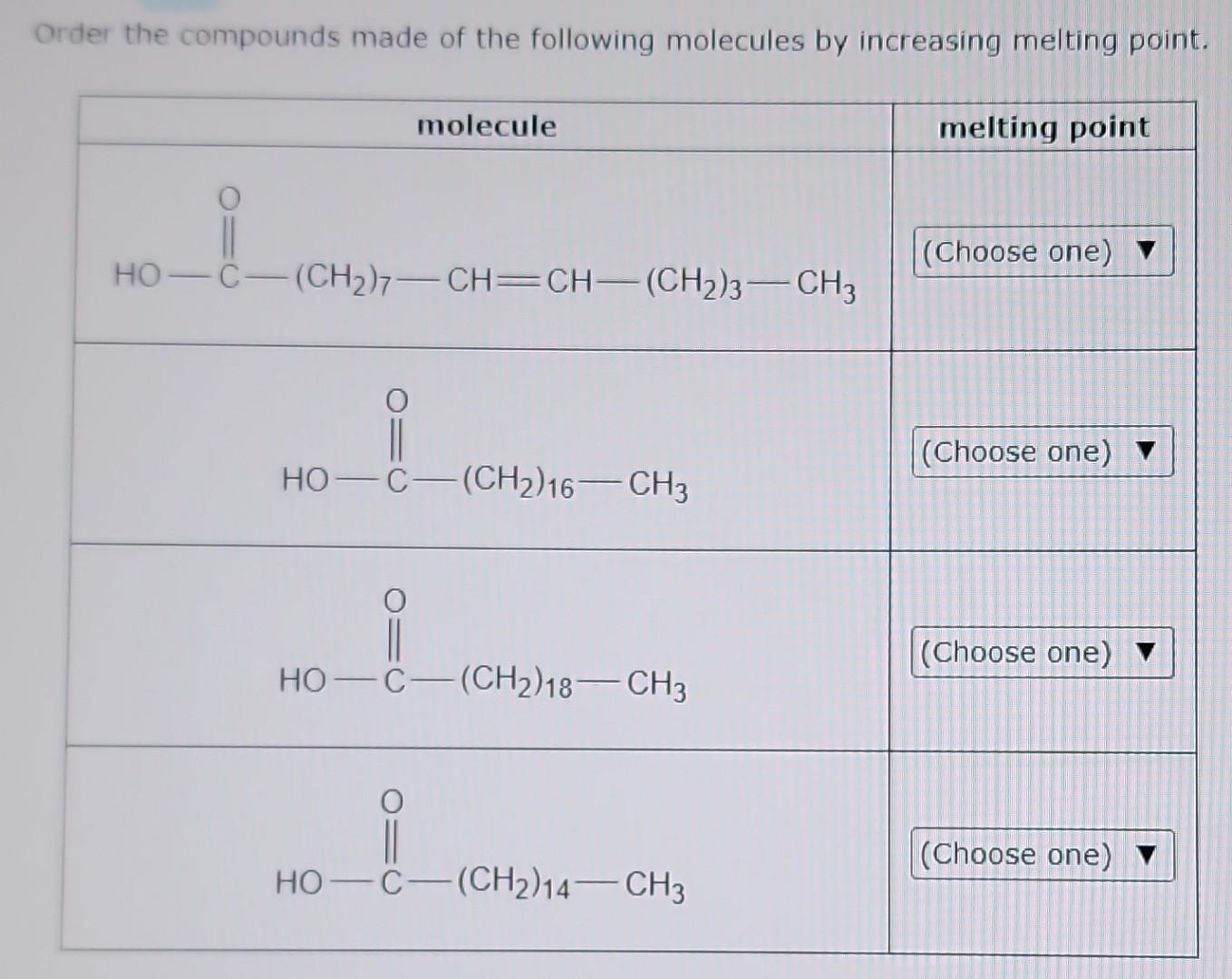
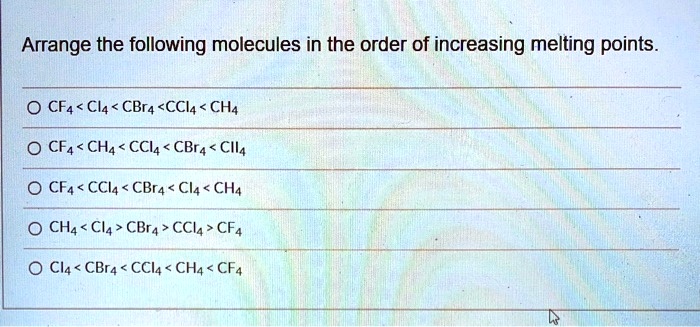
Arrange The Following Compounds In Order Of Increasing Melting Point

Alright, so imagine you're at a potluck, right? Everyone's brought their best dish. You've got your grandma's famous casserole (super dense, takes ages to heat up), your neighbor's light and fluffy cake (basically air, warms up in seconds), and then there's that weird, jiggly Jell-O mold from that one distant cousin you see once a decade. Now, if you were to put all these culinary creations into a slightly-too-warm oven and see how long it takes for them to reach "room temperature" (or, you know, stop being fridge-cold), you'd notice something pretty darn interesting. Some things melt, or soften, or just kind of… slump, way faster than others. This, my friends, is kind of like what chemists do when they're looking at how different compounds behave when you start cranking up the heat. We call this the melting point, and it's basically the temperature at which a solid decides it's had enough and goes, "You know what? I'm ready to mingle with my liquid buddies."
So, the challenge is this: we have a bunch of these chemical compounds, and we need to arrange them in order of their melting points, from the chilly ones that barely need a nudge to get going, all the way up to the stubborn ones that need a serious pep talk (or a blast furnace, but let's stick to slightly more accessible science here) to change their state. Think of it like lining up a bunch of friends for a race. Some are Usain Bolt, ready to sprint off the line. Others are more like me on a Monday morning – still trying to figure out which direction the finish line is, and needing a strong cup of coffee (or in this case, a lot of heat) just to get moving.
Now, the compounds we're looking at today are, in no particular order, methane, water, sodium chloride, and diamond. Sounds like a fun mixed bag, right? It's like a lineup of characters from different sci-fi movies. You've got your little guy, your essential life-giver, your salt-of-the-earth friend, and then, the ultimate boss. Each of these has its own personality when it comes to heat, and understanding why is where the fun begins.
The Great Melting Point Marathon: Who's Fast, Who's Feisty?
Let's break down our contenders. First up, we have methane. If methane were a person, it'd be that super-hyper kid who bounces off the walls and is practically vibrating with energy even at room temperature. It's a gas, meaning its particles are already having a grand old time, zipping around independently. To get methane to even think about becoming a liquid, you need to make it really, really cold. We're talking, like, “polar bear’s toenails” cold. So, its melting point is way down there in the negatives. It's the sprinter who’s already at the finish line before the starter pistol even fires. It needs very little encouragement to go from solid to liquid.
Next, we have water. Ah, water. The stuff of life, the reason we can make tea, the bane of our existence when it leaks from the roof. Water is fascinating because, at room temperature, it's already doing its liquid thing. But to get it to freeze into ice (which is just solid water), you need to chill it out. Its melting point is 0 degrees Celsius, or 32 degrees Fahrenheit. That's a pretty common temperature, right? It’s the temperature where you might start thinking about a light jacket, or when your iced coffee might actually start to melt faster than you can drink it. It's our steady jogger, not too fast, not too slow, but reliably doing its thing.
Then there's sodium chloride. Fancy name for good old table salt! Now, salt is a bit more… substantial than water. It's an ionic compound, meaning it's made of positively and negatively charged bits that are holding onto each other like a couple at a wedding who really don't want to let go. These ionic bonds are pretty strong, like a really tight hug. So, to get salt to melt, you need to apply a lot more heat than you do for water. We're talking, like, "oven on broil for an eternity" heat. Its melting point is way up there, around 801 degrees Celsius. This is our marathon runner who’s been training for years, needs serious endurance, and probably a packed lunch.
And finally, we have diamond. If diamond were a person, it would be the stoic, unmoving mountain. Diamonds are made of carbon atoms, all bonded together in this super-tough, three-dimensional lattice. These covalent bonds are some of the strongest known in chemistry. To get a diamond to melt, you'd need temperatures so high they'd make our salt friend look like it's having a spa day. We're talking, like, 3,550 degrees Celsius! That's hotter than the surface of the sun (okay, maybe not that hot, but close enough to make you sweat just thinking about it). Diamond is the one who’s practically glued to its spot, requiring an astronomical amount of energy to even consider changing its mind about being a solid.
Putting It All Together: The Grand Ranking
So, let's line them up. We want to go from the easiest to melt (lowest melting point) to the hardest to melt (highest melting point). It's like arranging your friends from "least effort to get out of bed" to "requires a personal intervention and possibly a crane."
First up, the undisputed champion of chill: methane. As a gas at room temperature, its solid form, methane ice, melts at a ridiculously low temperature of -182.5 degrees Celsius. It’s like that friend who’s always cold, even in summer, and needs a parka in July. But when it comes to melting, it’s the opposite – it melts at the lowest temperature, meaning it barely needs any warming up to become liquid. Its particles are already pretty far apart, and the forces holding them together in solid form are weak. Think of it as a loose handshake; easy to break.
Next in line is our good friend water. With a melting point of 0 degrees Celsius, it’s right there in the middle of our everyday experience. It’s the predictable one. You know when it’s going to freeze, you know when it’s going to melt. Its molecules, while they can form hydrogen bonds, are still relatively free to move compared to more rigid structures. It’s a firm handshake, requiring a bit more effort than methane’s casual wave, but still pretty easy to overcome.
Now, our seasoned runner, sodium chloride (salt). This guy is serious. With a melting point of a scorching 801 degrees Celsius, it's not messing around. Those strong ionic bonds, formed between the positive sodium ions and negative chloride ions, are like a super-glue job. It takes a significant amount of energy – a lot of heat – to pull those ions apart and let them flow as a liquid. Imagine trying to unstick two magnets that have been slammed together with all their might. It's a solid, dependable hug that takes serious effort to break free from.
And finally, the ultimate champion of stubbornness, the immovable object, diamond. Its melting point is a mind-boggling 3,550 degrees Celsius. This isn't just a strong bond; it's a covalent network where every carbon atom is sharing electrons with its neighbors in a rigid, almost unbreakable structure. It's like a family reunion where everyone is holding hands and forming a giant, impenetrable circle. You'd need the heat of a supernova to even think about melting this beast. It’s the ultimate endurance athlete, requiring an unbelievable amount of sustained effort to even budge.
Why Does This Matter, Anyway?
You might be thinking, "Okay, that's neat, but does this have anything to do with my life beyond explaining why my ice cubes disappear faster on a hot day?" Absolutely! Understanding melting points is super important in all sorts of practical applications. Think about cooking. Knowing the melting point of butter or chocolate helps you make perfect sauces and desserts. It's why you can't just throw a stick of butter into a boiling pot and expect it to melt instantly – it needs time to absorb that heat.
In materials science, knowing melting points helps engineers choose the right metals for everything from airplane engines to your car's radiator. They need materials that can withstand high temperatures without melting into a gooey mess. That’s why you don’t see car radiators made of ice, even though ice is pretty common! And it's why they don't use diamond in everyday plumbing – it's overkill, and frankly, impossible to work with for most applications.
Even in medicine, understanding how drugs melt can be important for how they are absorbed by the body. If a pill needs to melt to release its active ingredients, its melting point can be a crucial factor in its effectiveness. It’s all about controlling the state of matter, and temperature is the big player.
So, the next time you're dealing with something solid that's turning liquid – whether it's ice cream on a summer day, butter on a hot pan, or even just the frost melting off your windshield – you can give a little nod to the fascinating world of melting points. It’s a fundamental property that governs so much of how our world works, from the grandest celestial bodies to the simplest kitchen experiment. And it all boils down to how strongly those tiny particles in a compound want to stick together when the heat is on!
It’s a spectrum of resistance, a testament to the incredible diversity of how matter behaves. From the easily persuaded methane to the unyieldingly stoic diamond, each compound has its own unique temperature at which it surrenders to the liquid state. And in that surrender, we find a deeper understanding of the universe around us, one melting molecule at a time.
