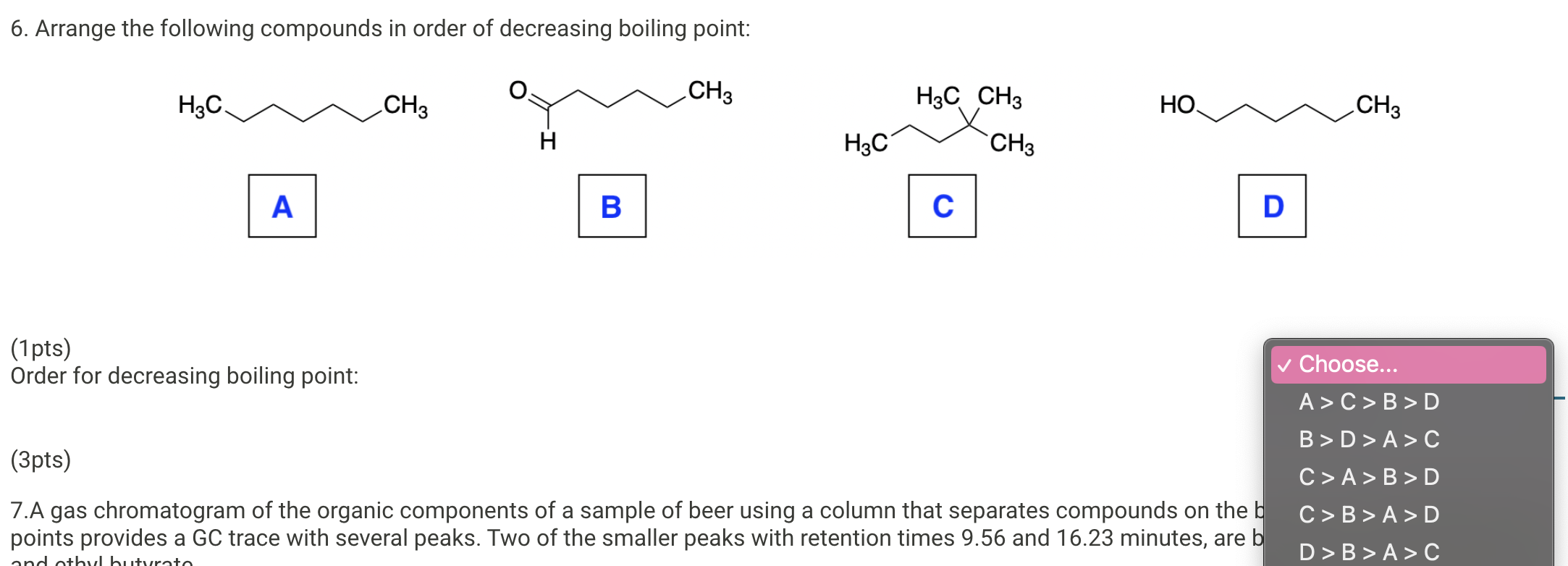
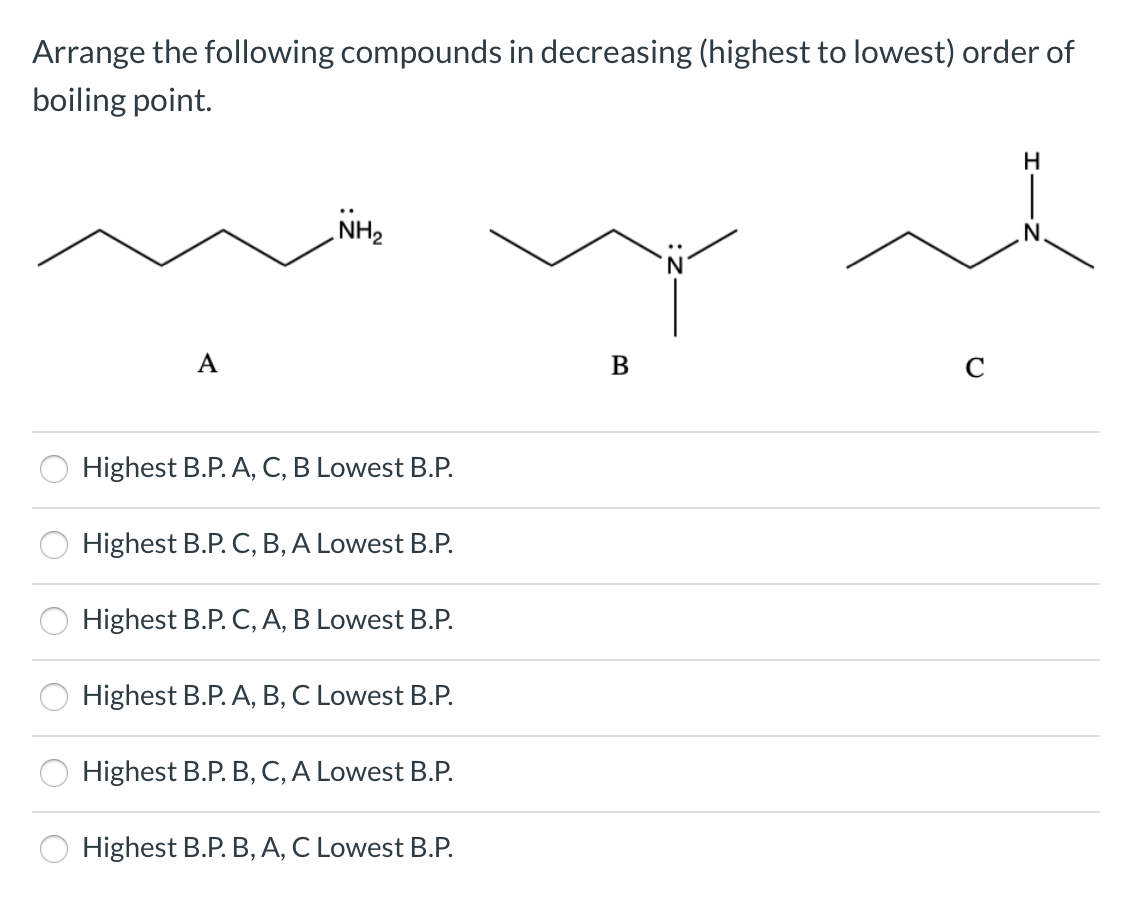
Arrange The Following Compounds In Order Of Decreasing Boiling Point
Hey there, fellow wanderers! Ever found yourself staring at a shelf of interesting liquids, maybe in your kitchen or even a science kit you unearthed from your childhood, and wondered, "What makes this one simmer before that one?" It’s all about the fascinating dance of molecules, and today, we're going to waltz through the world of boiling points. Think of it like a super chill gathering of microscopic buddies – some are more eager to break free and float around than others. We're talking about arranging a few compounds, and trust me, it’s way more exciting than it sounds. Forget stuffy textbooks; we're channeling our inner cool science guru, with a side of latte and good vibes.
So, what exactly is a boiling point? In the simplest terms, it's the temperature at which a liquid turns into a gas. It’s that magical moment when all those little molecules decide they’ve had enough of holding hands and want to go their own way, floating freely in the air. It’s like when your favorite song comes on and you just have to get up and dance – the molecules are doing their own version of that, just with a lot more heat involved.
The compounds we're going to play with today are: water (you know this one!), ethanol (hello, happy hour!), hexane (think lighter fluid, but let's keep it sophisticated), and methane (the main ingredient in natural gas, super simple!). Our mission, should we choose to accept it, is to order them from highest boiling point to lowest. It’s a bit like ranking your favorite pizza toppings – everyone has their opinion, but there’s a general consensus, and understanding why is the fun part.
The Secret Ingredient: Intermolecular Forces
What dictates how eager those molecules are to mingle or make their escape? It’s all about the intermolecular forces. Think of these as the invisible glue holding the molecules together. The stronger the glue, the more energy (heat!) you need to break them apart and make them boil. It's like trying to pull apart a group of friends who are in a really tight hug versus those who are just loosely holding hands.
There are a few main types of these molecular embraces. We’ve got London dispersion forces, which are like fleeting little whispers of attraction. They’re present in all molecules, but they’re strongest when molecules are bigger and have more electrons bouncing around. Then there are dipole-dipole interactions, which are a bit more like friendly waves – slightly stronger attractions between molecules that have a permanent positive and negative end. And finally, the heavyweight champion of intermolecular forces: hydrogen bonding. This is like a super-strong, dedicated handshake, and it happens when hydrogen is bonded to a very electronegative atom like oxygen, nitrogen, or fluorine. It’s a big deal!
So, to figure out our boiling point order, we need to look at which of our compounds has the strongest intermolecular forces. It’s a bit like a cosmic popularity contest, but with science.
Our Contenders: A Closer Look
Let's meet our contestants:
- Methane (CH₄): This is the simplest hydrocarbon. It’s a small, symmetrical molecule. Think of it as the baby of the group, super lightweight and not much going on in terms of complicated interactions. Its primary intermolecular forces are just those fleeting London dispersion forces.
- Hexane (C₆H₁₄): This is a bigger hydrocarbon than methane, with six carbon atoms and fourteen hydrogen atoms. It’s a longer chain. Because it's bigger and has more electrons, its London dispersion forces will be stronger than methane's. More electrons mean more opportunities for those little temporary charge imbalances that create attraction.
- Ethanol (C₂H₅OH): This is the alcohol in your favorite vino or craft beer. It has a carbon chain, but importantly, it also has an –OH group (that's an oxygen bonded to a hydrogen). This –OH group is the magic wand for hydrogen bonding!
- Water (H₂O): The universal solvent and the essence of life! Water has two –OH groups. This means it can form lots of hydrogen bonds. It’s like a molecule that’s a natural-born hugger, capable of forming multiple strong connections with its neighbors.
The Big Reveal: Ranking Our Compounds
Now, let's put it all together and get our ranking. Remember, stronger intermolecular forces = higher boiling point. It's like needing more effort to unstick something that's really, really stuck.
The Winner (Highest Boiling Point): Water (H₂O)
Why? Water is the undisputed champion here. It’s capable of extensive hydrogen bonding. Each water molecule can form up to four hydrogen bonds with its neighbors – it’s a true social butterfly at the molecular level! This strong network of hydrogen bonds requires a huge amount of energy to break, hence its relatively high boiling point of 100°C (212°F) at standard atmospheric pressure. It's why we can boil water on the stove, and it doesn't just instantly vanish into thin air like some lighter substances might.
Fun Fact: Did you know that life as we know it wouldn't exist without water's high boiling point? It allows oceans and lakes to exist in liquid form, providing a stable environment for countless species. Plus, it makes for a great cup of tea on a chilly morning!
Second Place: Ethanol (C₂H₅OH)
Why? Ethanol also participates in hydrogen bonding because of its –OH group. However, it's not as extensive as in water. The presence of the nonpolar ethyl group (C₂H₅) can interfere slightly with the optimal arrangement for hydrogen bonding compared to water's compact structure. It still has significant attractions, but not quite the same density of hydrogen bonds as water. Ethanol boils at around 78.37°C (173.07°F). It's a good jump up from our next contenders, thanks to that hydrogen bonding!
Cultural Connection: Ethanol is the alcohol found in alcoholic beverages. Its boiling point is crucial for distillation, the process used to concentrate alcohol and create spirits like vodka, whiskey, and rum. Cheers to science!
Third Place: Hexane (C₆H₁₄)
Why? Hexane doesn't have any oxygen or nitrogen atoms bonded to hydrogen, so it cannot form hydrogen bonds. Its intermolecular forces are solely London dispersion forces. However, hexane is a much larger molecule than methane. This increased size and number of electrons means its London dispersion forces are significantly stronger than those in methane. More electrons = more "stickiness." Hexane boils at about 68.7°C (155.7°F). It's pretty close to ethanol's boiling point, but the lack of hydrogen bonding makes it lower.
Practical Tip: You might encounter hexane in laboratories as a solvent. It's good for dissolving nonpolar substances. Just remember, while its boiling point isn't extremely high, it is flammable, so always handle with care and in a well-ventilated area. Think of it as a more volatile cousin of the cooking oils you use!
The Winner (Lowest Boiling Point): Methane (CH₄)
Why? Methane is the smallest molecule here. It's tiny, and its electron cloud is not very extensive. Therefore, it has the weakest London dispersion forces of our group. Without hydrogen bonding or significant dipole-dipole interactions, it takes very little energy for methane molecules to overcome these weak attractions and become a gas. Methane boils at a frigid -161.5°C (-258.7°F). This is why natural gas, which is mostly methane, is stored and transported as a compressed gas under pressure, even at relatively low temperatures.
Fun Fact: Methane is a greenhouse gas, and it's also produced naturally by cows digesting their food! So, next time you're enjoying a burger, you're indirectly contributing to the methane cycle. Earth is a wild, interconnected place.
Putting It All Together: The Order
So, our order of decreasing boiling point, from highest to lowest, is:
- Water (H₂O) - Highest boiling point due to extensive hydrogen bonding.
- Ethanol (C₂H₅OH) - High boiling point due to hydrogen bonding, but less extensive than water.
- Hexane (C₆H₁₄) - Moderate boiling point due to stronger London dispersion forces in a larger molecule.
- Methane (CH₄) - Lowest boiling point due to weak London dispersion forces in a small molecule.
It’s a beautiful hierarchy, driven by the subtle yet powerful forces that govern how molecules interact. It’s like a dance floor: water is having a full-on rave with hydrogen bonds, ethanol is enjoying a lively party with some hydrogen bonds, hexane is doing a more relaxed shuffle with stronger dispersion forces, and methane is just doing a solo, minimalist groove with barely any pull.
Beyond the Beaker: Connecting to Our Lives
Why does any of this matter outside of a chemistry lab? Well, understanding boiling points is surprisingly relevant to our everyday lives! Think about cooking. When you boil water for pasta or to make tea, you're operating at its boiling point. The fact that it has a manageable boiling point is what makes it so useful.
Consider refrigeration. The refrigerants used in your fridge and air conditioning systems are specifically chosen for their boiling points. They need to boil at very low temperatures to absorb heat from the inside of your fridge, effectively cooling it down. Then, they can be compressed and condensed back into a liquid, ready to start the cycle again.
Even something as simple as choosing a perfume or cologne involves understanding volatility, which is related to boiling points. Lighter, more volatile compounds (lower boiling points) evaporate quickly, giving you that initial burst of fragrance, while heavier, less volatile compounds (higher boiling points) linger longer.
And when you're enjoying a relaxing bath, the steam rising is water vapor, a direct result of water reaching its boiling point. It’s a constant, gentle reminder of these molecular interactions happening all around us.
A Moment of Reflection
It’s easy to think of science as something distant, confined to sterile labs with glowing tubes. But the truth is, the principles that govern molecules are at play in every breath we take, every meal we cook, and every drop of rain that falls. The order of boiling points for these simple compounds – water, ethanol, hexane, and methane – is a tiny window into this vast, intricate universe. It reminds us that even the most ordinary substances have fascinating stories to tell, and that the world around us is a constant, dynamic dance of invisible forces. So next time you’re enjoying a hot cup of coffee or feeling the cool breeze on your face, take a moment to appreciate the molecular magic that makes it all possible. It’s a wonderfully complex world, beautifully and simply orchestrated by attraction.
