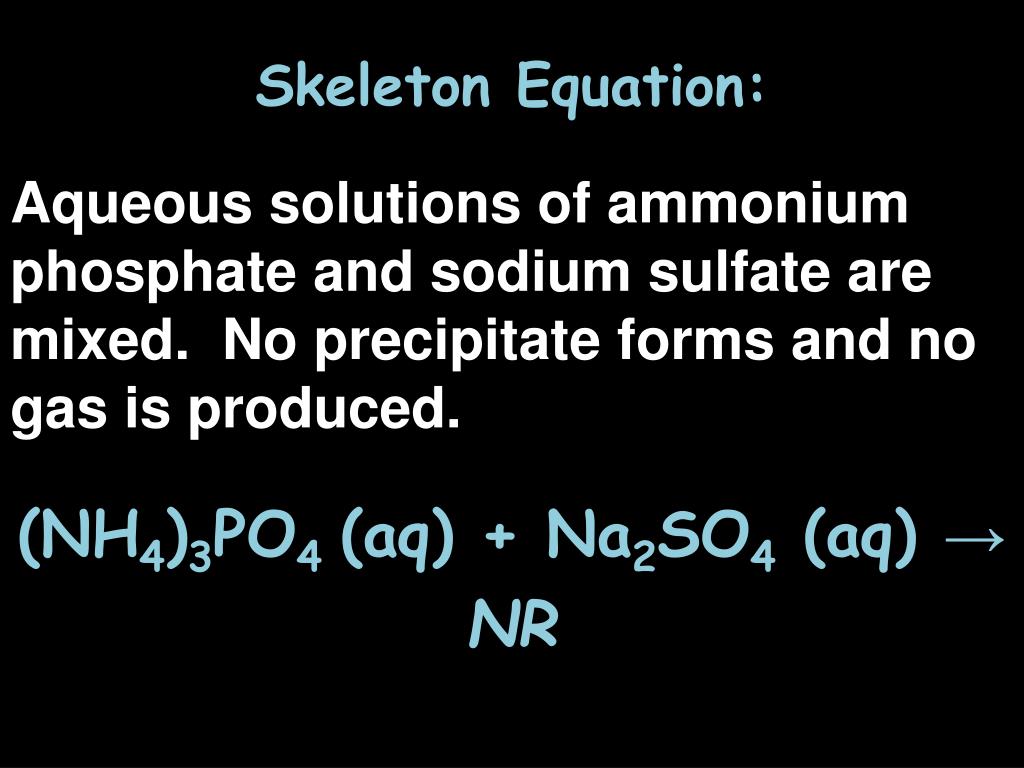
Aqueous Solutions Of Ammonium Phosphate And Sodium Sulfate Are Mixed
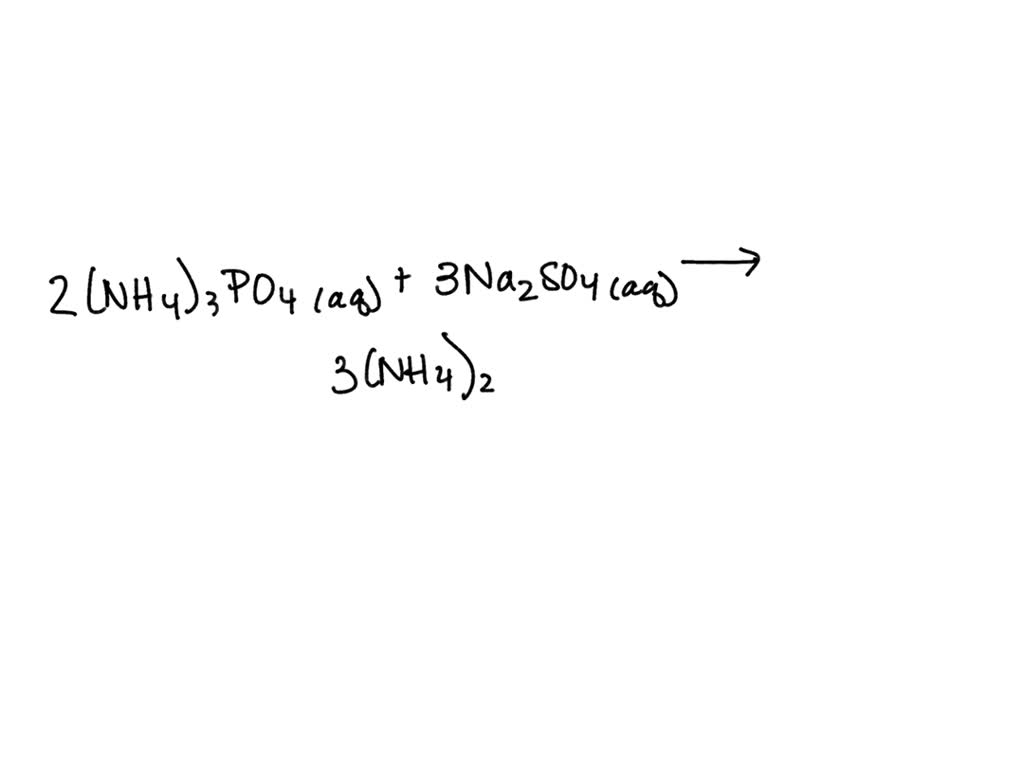
Life, in its wonderfully chaotic dance, often brings together elements that seem entirely unrelated. Think of that perfect playlist for a road trip, or the unexpected friendship that blossoms over a shared love for obscure indie films. Well, today we're diving into a similar kind of harmonious collision, but in the realm of chemistry. Prepare yourself for the delightful mingling of aqueous solutions of ammonium phosphate and sodium sulfate. Don't let the scientific jargon trip you up; at its heart, this is a story about how different ingredients can create something entirely new, and often, something quite useful. It’s less about complex reactions and more about the subtle, sometimes surprising, outcomes that emerge when worlds collide.
Imagine you're in your kitchen, whipping up a storm. You’ve got your baking soda, your vinegar, maybe some food coloring for that extra flair. You know that when they meet, there’s a fizz, a transformation. This chemical tango between ammonium phosphate and sodium sulfate is a bit like that, though the "fizz" might be less dramatic and more… practical. These aren't just random chemicals; they're common players in various industries, and understanding their interaction is like understanding the secret ingredients in your favorite products, or even the subtle processes that keep our world running smoothly.
The Cast of Characters
Let's get acquainted with our main actors. First up, we have ammonium phosphate. This is a family of salts, typically formed from ammonia and phosphoric acid. Think of it as a dual-purpose performer. Depending on the ratio of ammonia to phosphoric acid, you can get monammonium phosphate (MAP), diammonium phosphate (DAP), or triammonium phosphate. These guys are superstars in the world of fertilizers, providing essential nutrients for plant growth. They’re the reason your garden veggies are so plump and your lawn so vibrantly green. Beyond agriculture, they also pop up in fire retardants and even as food additives – imagine them subtly enhancing the texture of that cookie you’re craving.
On the other side of the stage, we have sodium sulfate. This is another salt, composed of sodium and sulfate ions. It's a bit of a workhorse, appearing in everything from laundry detergents to glass manufacturing. Its primary role is often as a filler or a processing aid. In detergents, it helps to prevent the powder from clumping and improves its dissolving properties. In glassmaking, it acts as a flux, lowering the melting point of silica. It’s the unsung hero, the reliable supporting actor that makes the magic happen behind the scenes. So, these two, ammonium phosphate and sodium sulfate, are not strangers to our daily lives, even if we don't always realize it.
The Grand Unveiling: What Happens When They Meet?
When these two aqueous solutions – meaning they're dissolved in water, like dissolving sugar in your tea – are mixed, a fascinating, albeit often understated, chemical ballet begins. It's like introducing two different groups of friends at a party; at first, there might be a bit of polite mingling, and then, connections start to form. In this case, the ions present in each solution start to interact. We have ammonium ions ($NH_4^+$) and phosphate ions ($PO_4^{3-}$) from ammonium phosphate, and sodium ions ($Na^+$) and sulfate ions ($SO_4^{2-}$) from sodium sulfate. These ions are like tiny, energetic dancers, constantly moving and looking for new partners.
The magic, or rather the science, here lies in solubility. When you mix these solutions, the ions can essentially "swap partners." The question becomes: are these new pairings stable and soluble in water, or do they form something that precipitates out, creating a solid? For the most part, when you mix ammonium phosphate and sodium sulfate in typical concentrations, the resulting ions – ammonium sulfate ($(NH_4)_2SO_4$) and sodium phosphate ($Na_3PO_4$) – remain happily dissolved in the water. They're all good swimmers, so to speak, and continue to exist as separate ions within the solution. This is a key point: no dramatic precipitate usually forms under normal conditions.

Think of it like a carefully curated playlist. You've got your upbeat pop tracks (sodium ions and sulfate ions) and your more mellow indie tunes (ammonium ions and phosphate ions). When you blend them, you don't end up with silence or a jarring noise; you get a richer, more complex soundscape. The individual melodies are still there, but they now coexist, creating a unique listening experience. Similarly, the ions are still present, but now they’re part of a larger, more intricate aqueous environment.
Practical Applications and Everyday Echoes
So, why should you care about this particular chemical encounter? Because understanding these simple interactions can shed light on a surprising number of everyday phenomena and industrial processes. Remember those fertilizers we talked about? Ammonium phosphates are crucial for plant nutrition. When they're manufactured, or when they're applied to the soil, they're interacting with all sorts of other substances, including sulfates that might be naturally present or added for other reasons. The fact that they generally remain soluble means that these nutrients can be effectively absorbed by plants.
Consider the world of cleaning. Sodium sulfate is a common ingredient in laundry detergents. While it doesn't directly clean, it helps the detergent work better by improving its solubility and preventing clumping. Ammonium phosphates, on the other hand, can be used in some specialized cleaning agents due to their ability to break down grease and other organic matter. The potential for these chemicals to coexist and not interfere negatively with each other's intended functions is crucial for product formulation. It’s like designing a multi-tool; each component needs to work effectively without compromising the others.

The agricultural industry is a prime example. Farmers often use complex fertilizer blends. These blends are carefully designed to provide a spectrum of nutrients. Understanding how different components, like phosphates and sulfates, interact – or rather, don't negatively interact – is fundamental to creating effective and stable fertilizer products. Imagine the frustration if adding one essential nutrient caused another to become unusable or to form a useless solid at the bottom of the fertilizer bag! It’s a testament to the elegance of chemistry that these compounds can coexist and serve their purpose.
Fun Facts and Chemical Curiosities
Did you know that ammonium phosphate has a fascinating history? It was one of the early breakthroughs in synthetic fertilizers, significantly boosting agricultural yields and helping to feed a growing global population. It's a real-world example of science directly impacting human well-being on a massive scale. And speaking of scale, the sheer amount of sodium sulfate produced annually is staggering – millions of tons! It’s a testament to its versatility and its quiet but vital role in so many industries. It’s the kind of chemical that doesn't always get the spotlight, but without it, many of the products we rely on wouldn’t be as effective or as affordable.
Here's a little chemical trivia for your next dinner party: The term "aqueous solution" itself is a nod to the Latin word "aqua," meaning water. So, whenever you hear "aqueous," just think "dissolved in water." It’s a simple connection that makes the scientific jargon a little less intimidating. And ammonium phosphate isn't just for plants; it's also a key ingredient in some of the most effective fire extinguishers! When heated, it decomposes and releases ammonia gas, which smothers flames. Talk about a multi-talented molecule!
The interaction between ammonium phosphate and sodium sulfate isn't about creating a dramatic new substance that explodes or changes color wildly. It's more about a subtle compatibility, an understanding between ions that allows them to share the same watery space. It’s like a well-choreographed dance where the dancers move around each other gracefully, each maintaining their individual rhythm while contributing to the overall flow. The absence of a precipitate is, in this context, a positive outcome. It means the components remain in a form that can be readily used for their intended purposes.

A Touch of Culinary Chemistry
Even in the kitchen, you can find echoes of these chemical principles. While you won't be mixing ammonium phosphate and sodium sulfate directly, think about how different ingredients interact. When you bake a cake, flour, sugar, eggs, and leavening agents (like baking soda, which is sodium bicarbonate) all come together. The leavening agent reacts with acidic components in the batter to produce carbon dioxide gas, causing the cake to rise. The other ingredients, like flour and sugar, provide structure and sweetness. It's a complex interplay where each component plays a role, and the success of the final product depends on their compatibility.
Similarly, when you're making a savory stew, you add various spices and seasonings. Salt (sodium chloride) enhances flavor, herbs add aroma, and perhaps you add a thickening agent like cornstarch (a carbohydrate). These ingredients don't typically react in a way that forms a new solid; instead, they meld together to create a delicious and cohesive dish. The principles of solubility and ion interaction, even if not explicitly named, are at play in creating the harmonious flavors and textures we enjoy.
The Bigger Picture: Interconnectedness
The seemingly simple act of mixing aqueous solutions of ammonium phosphate and sodium sulfate is a micro-level illustration of a much larger principle: interconnectedness. Our world is a vast network of chemical, biological, and social systems, and the way different components interact, or coexist, is fundamental to how everything functions. From the microscopic world of ions to the macroscopic world of ecosystems and economies, understanding these interactions is key to progress and sustainability.
This is why chemists spend so much time studying reaction conditions, solubility, and compatibility. It’s about ensuring that the elements we use can work together harmoniously to achieve desired outcomes, whether that’s a higher crop yield, a more effective detergent, or a safer environment. It’s a constant quest for balance and efficiency.
Think about your own life. You’re a blend of different experiences, relationships, and skills. Sometimes, these elements clash, and sometimes they complement each other beautifully, creating a unique and fulfilling life. The way you navigate your friendships, your career, and your personal growth is all about how different aspects of your life interact. Just like ammonium phosphate and sodium sulfate finding their place in the same solution, you find your equilibrium by allowing different parts of yourself and your life to coexist and enrich each other.
So, the next time you hear about the mixing of aqueous solutions of ammonium phosphate and sodium sulfate, don't just picture beakers and bubbling liquids. Picture the fertilizers that nourish our crops, the detergents that keep our clothes clean, and the vast, interconnected tapestry of chemistry that underpins so much of our modern world. It’s a reminder that even the most seemingly obscure scientific interactions can have profound and far-reaching implications, often in ways we never even notice.
And in the end, isn't that what life is all about? Discovering the unexpected harmony in different elements, understanding how they interact, and appreciating the beautiful, complex solutions that emerge. Just like these two chemical compounds, we too are constantly mixing, reacting, and finding our place in the grand aqueous solution of existence.
