An Electron Dot Structure Is A Convenient Method Of Representing

Imagine you're at a huge family reunion, and everyone's trying to introduce themselves. It's a beautiful chaos, right? You've got your Aunt Mildred, your cousin Steve, and a whole bunch of people you've never met before. Now, imagine if each person had a little "intro card" that just showed their most important family members. That's kind of like what an electron dot structure is for atoms!
It's a super handy way to get a quick snapshot of an atom's personality, specifically the parts that are ready to mingle and make new friends. Think of it as a simplified social media profile for these tiny building blocks of everything around us. They don't show everything, just the bits that are most interesting for how atoms behave.
These "dots" aren't actual glitter bombs or anything as exciting as that. They represent the valence electrons. These are the electrons hanging out on the outermost shell, like the kids on the playground who are always up for a game. They're the ones who get to interact and form connections.
It's like a game of "dot-to-dot" but for science! You draw the symbol of the element, like a little name tag, and then you place these dots around it. Each dot is like one of those outer-shell electrons just waiting to be noticed. It’s a visual shorthand, a quick way to see who’s got how many "friends" ready to be made.
Think about carbon, the backbone of life! It's got four valence electrons. So, its electron dot structure looks like the letter 'C' with four dots scattered around it. It’s like saying, "Hey, I'm Carbon, and I'm ready to bond with four other things!" It's no wonder it forms such complex molecules; it's got a lot of potential friends to make.
Then there's oxygen. It's like the slightly more reserved friend, with six valence electrons. Its structure shows the 'O' with six dots. It's often looking to grab a couple more electrons to feel complete, which is why it's so eager to bond with things like hydrogen to make, you guessed it, water! H₂O, the stuff of life!

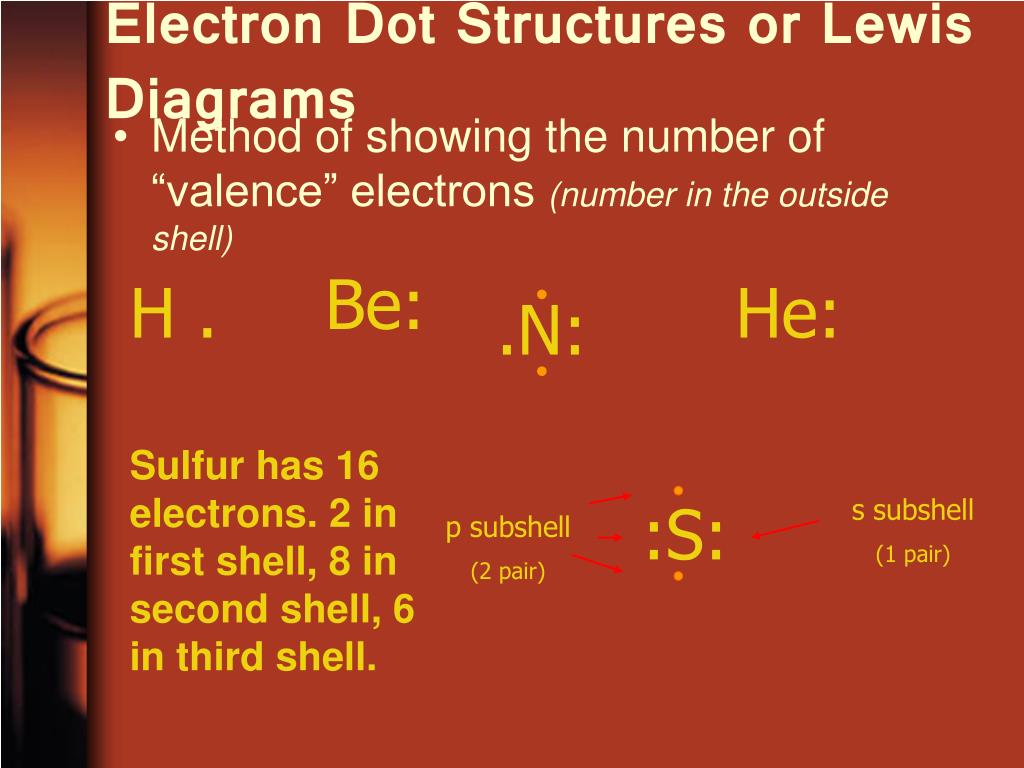
And what about helium? It’s that cool, aloof character. It only needs two electrons to feel perfectly content. Its dot structure is just 'He' with two dots, usually snuggled up together. It’s happy being on its own, which is why helium balloons float so serenely. It's a noble gas, meaning it doesn't really bother with making friends.
It's truly a heartwarming thought, isn't it? These tiny, invisible entities are always seeking connection, a sense of completeness. The electron dot structure is like a little window into their atomic social lives, showing their desire to pair up, share, or even take from others. It's a silent ballet of attraction and repulsion.
Consider sodium. It's the generous type, with just one lone valence electron. Its dot structure is a 'Na' with a single dot. It's practically begging to give that electron away to someone who needs it more, forming a strong bond with elements like chlorine, which is craving just one electron.
And chlorine! It’s like the person who always has room for one more guest. It has seven valence electrons. Its structure shows a 'Cl' with seven dots. When sodium offers its electron, chlorine is overjoyed, and they form sodium chloride – that’s right, regular old table salt! It's a beautiful, if slightly salty, friendship.
These structures are so useful because they immediately tell us about an atom's reactivity. The more dots you have, the more possibilities there are for bonding. It's like looking at someone's resume – you can quickly get a sense of their experience and potential.
It’s a simplified representation, of course. The real world of electrons is a bit more complex, like a bustling city with everyone moving at lightning speed. But for understanding the basics of how atoms interact and form the molecules we see and touch every day, the electron dot structure is like having a friendly neighborhood guide.
It’s also a fantastic tool for students learning about chemistry. Instead of memorizing complex molecular formulas right away, they can draw these simple diagrams and see the connections visually. It’s like learning to read by looking at picture books first. The dots make abstract concepts tangible and understandable.

Sometimes, when you look at a complex molecule built from many atoms, and you see all those shared dots, it's like a map of a collaborative effort. Each atom contributing its valence electrons to form a stable, larger structure. It's a testament to how even the smallest particles work together to create the universe.
Think of a water molecule again. You see the 'O' with its two lone pairs of electrons and the two 'H' atoms each with their single electron. It’s a little visual story of sharing and stability. The oxygen atom shares its electrons with two hydrogen atoms, and in return, the hydrogen atoms share theirs. They all end up feeling more "complete."
This method was popularized by chemists like Gilbert N. Lewis. He was like the architect of these atomic "friendship diagrams." He realized that by focusing on these outermost electrons, we could unlock a lot of the secrets of chemical bonding. It was a brilliant simplification that changed how we understood the chemical world.
It's a reminder that even in the most fundamental aspects of science, there's elegance and a certain charm. The electron dot structure isn't just a scientific tool; it's a visual poem about connection, stability, and the fundamental drive of matter to interact and form something new.

So, next time you hear about an electron dot structure, don't just think of dots on a page. Think of it as the atom’s way of saying, "Here’s who I am and who I’m looking to connect with!" It’s a simple, yet profound, way to understand the building blocks of everything. It's a little glimpse into the secret social lives of atoms, and frankly, it's pretty amazing.
It’s like learning the secret handshake of the atomic world. Once you know the dots, you can start to understand why certain atoms stick together and others prefer to remain solitary. It’s the fundamental language of chemistry, written in dots and symbols. And once you learn it, the world of molecules opens up in a whole new, fun way.
It’s a bit like recognizing patterns in a crowd. You see a group of people with similar shirts, and you know they’re part of the same team. Similarly, when you see a certain arrangement of dots around an atom, you know something about its potential to join forces and create something wonderful, like a plastic bottle, a delicious cookie, or even the air you breathe.
It's a visual representation that makes complex ideas feel surprisingly approachable and, dare I say, even charming. It’s the atom’s way of putting its best foot forward.
So, the next time you encounter an electron dot structure, give it a little nod. It's a testament to human ingenuity in simplifying the incredibly complex, and a beautiful reminder that even the smallest things are driven by a desire to connect and form something greater than themselves. It’s a tiny, dot-filled window into the vast and intricate universe of chemistry.
