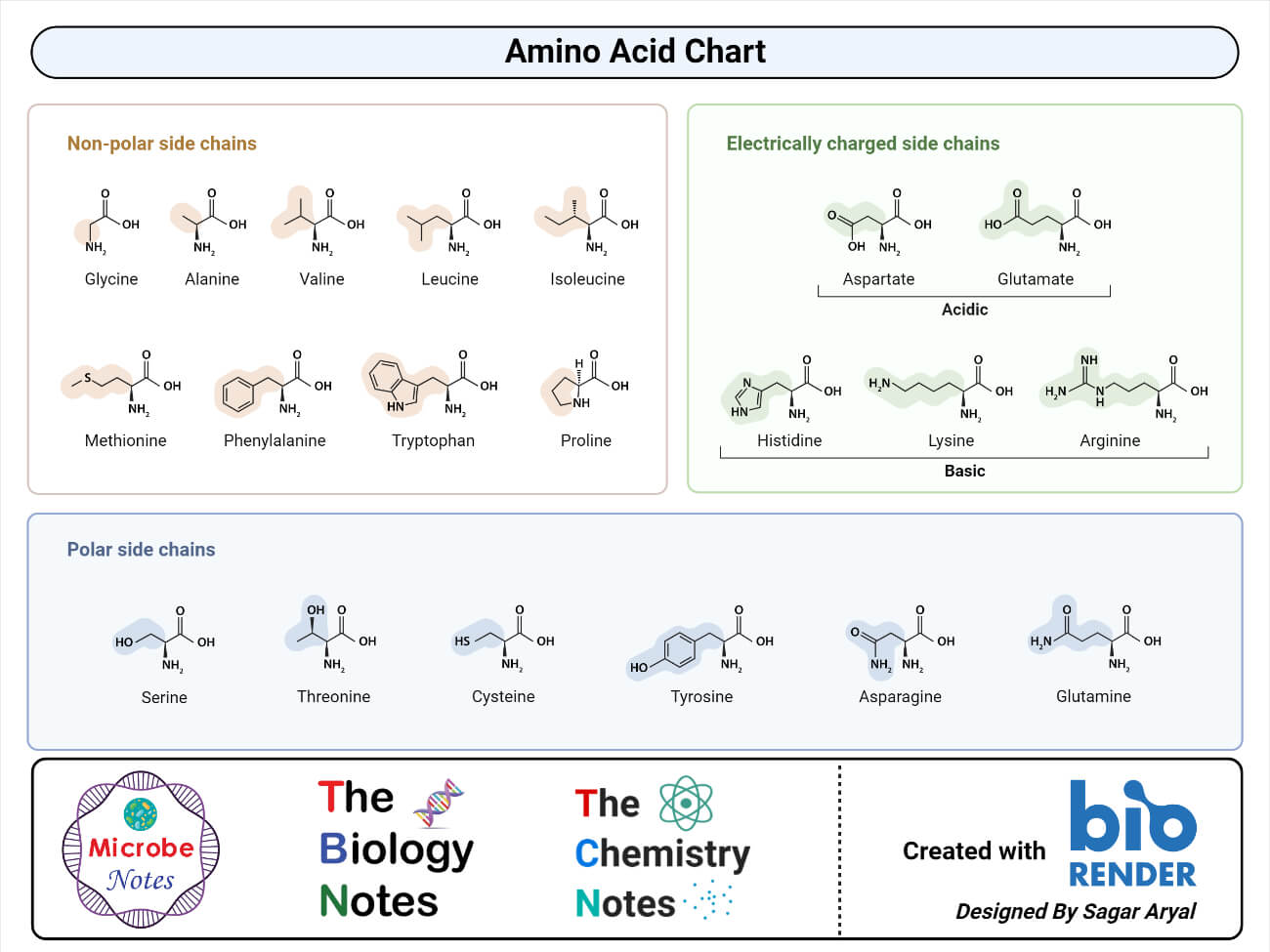
Amino Acids Are Acids Because They Possess Which Functional Group

Hey there, science curious folks! Ever wondered why something with a name like "amino acid" is, well, an acid? It's not some sneaky trick. It's all about the tiny, mighty bits and bobs that make them tick. And guess what? We're gonna dive into that, and it's gonna be fun. No boring lectures here, promise!
Think of amino acids as the building blocks of everything. Seriously, everything. Your muscles? Amino acids. Your hair? Amino acids. That amazing feeling of happiness? Yep, amino acids are involved.
So, what's the big deal about them being "acids"? It all comes down to a super important little group of atoms. The one that gives them their acidic superpower. It’s a bit of a mouthful, but stay with me!
The Secret Ingredient: The Carboxyl Group!
So, the main reason amino acids are considered acids? It’s because they’ve got a special guest hanging out. A little chemical crew. This crew is called the carboxyl group.
Imagine a party. You’ve got a bunch of guests, right? The carboxyl group is like the guest that brings all the fizzy drinks. It makes things a little zesty, a little tangy. That's the acidic part.
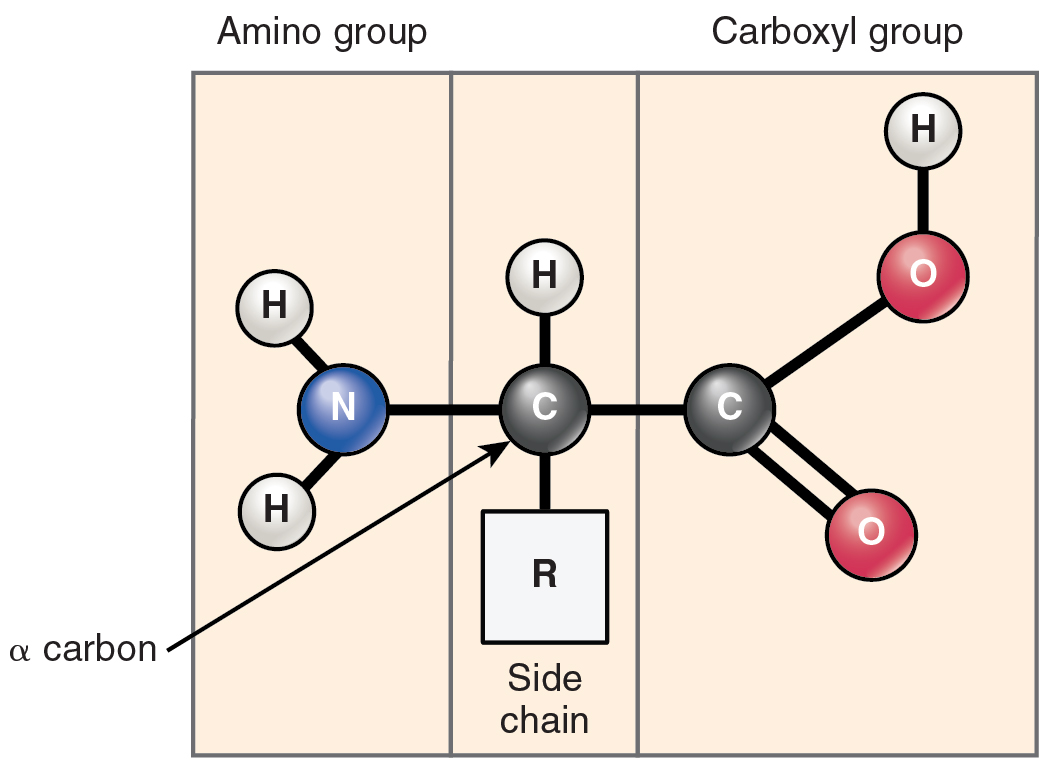
What does this carboxyl group look like? It’s a carbon atom, chilling with two oxygen atoms. And one of those oxygen atoms is also holding hands with a hydrogen atom. So, it's like -COOH. Fancy, huh?
This little -COOH is the star of the show when it comes to acidity. Why? Because it's got this hydrogen atom that's just itching to leave. It’s like a kid who can't wait to run off and play. This hydrogen atom is a bit positive, and it likes to share itself.
![[ANSWERED] Amino acids are acids because they possess which functional](https://media.kunduz.com/media/sug-question-candidate/20220511035551351008-4536923.jpg?h=512)
When this hydrogen atom bounces off, it leaves the rest of the carboxyl group with a negative charge. And that, my friends, is the hallmark of an acid! It's all about that ability to donate a proton. That proton is just a fancy word for that hydrogen atom on the move.
So, every amino acid has at least one of these carboxyl groups. It’s like their badge of honor. Their little acid signature. Without it, they’d just be… well, something else entirely. And we wouldn’t be talking about them as amino acids!
But Wait, There's More! The Amino Group!
Now, here’s where it gets really interesting. Amino acids aren't just acids. They're also… well, amino! And that comes from another super cool functional group. You guessed it: the amino group!
This amino group is like the yin to the carboxyl group's yang. It’s what makes them unique and incredibly versatile. The amino group is basically a nitrogen atom hanging out with two hydrogen atoms. Think -NH2. Also pretty neat!
And here’s the quirky part: the amino group is actually the opposite of acidic! It’s a base. It’s like the guest who brings all the comfy cushions to the party. It’s got a bit of a tendency to *accept protons. It’s a proton- acceptor, not a proton-donor.
So, you have this one molecule, an amino acid, that has both an acidic part (the carboxyl group) and a basic part (the amino group). Mind. Blown.
The Zwitterion Shenanigans!
This dual personality, having both an acid and a base in the same molecule, leads to some seriously cool chemistry. It allows amino acids to exist in a special form called a zwitterion. Isn’t that a fun word to say? Zwitterion!
In a zwitterion, the carboxyl group has donated its hydrogen atom (so it's negatively charged), and the amino group has grabbed a hydrogen atom (so it's positively charged). So, the whole molecule ends up being electrically neutral overall, but it has these little positive and negative ends. It's like a tiny, perfectly balanced seesaw!
This zwitterionic form is super important for how amino acids behave in different environments, especially in water. It’s why they can dissolve and do all the amazing things they do in our bodies.

It’s this amazing ability to act as both an acid and a base that makes amino acids so central to life. They can buffer pH changes, which is crucial for keeping our cells happy and healthy. They are the ultimate multitaskers!
Why Is This So Fun to Talk About?
Okay, so why is this little bit of chemistry so darn fun? For starters, the names are just inherently cool. Carboxyl group. Amino group. Zwitterion. They sound like characters from a quirky sci-fi movie. Or maybe secret agents.
And it's the unexpected dual nature that’s so captivating. We think of acids as, you know, sour and stingy. And bases as slippery and a bit… well, basic. But here we have molecules that are both? It’s like discovering your quiet neighbor is secretly a rockstar.
Plus, it’s the foundation of so much. Understanding amino acids and their functional groups is like getting the cheat codes to understanding proteins, enzymes, DNA, and basically, the recipe for life itself. That's pretty epic!
Think about it. Every single protein in your body is just a long chain of these amino acid building blocks, linked together in a specific order. The sequence, dictated by your DNA, determines the protein's shape and its function. It’s like an alphabet, but instead of letters, you have these amino acids, and they spell out everything your body needs to do.
And the "acid" part is just the starting point. It’s the characteristic that allows them to join up, to form those peptide bonds that create proteins. It’s the chemical handshake that links them together, one after another, building the incredible structures that keep us alive and kicking.
It’s also a great reminder that things aren’t always what they seem on the surface. A molecule named "amino acid" has both acidic and basic properties. It’s a testament to the beautiful complexity and sometimes surprising nature of chemistry. It’s the little details that make the big picture so fascinating.
So, next time you hear about amino acids, you can nod knowingly. You know their secret. You know about the carboxyl group, that little -COOH that makes them acids. And you know about their amino group, the -NH2 that makes them bases. And you know about the zwitterion, their cool, neutral alter-ego. It's all about those functional groups!
It’s a simple concept at its core, but the implications are massive. It’s the science of life, broken down into its most fundamental components. And honestly? That’s pretty darn cool. So go forth, and impress your friends with your newfound knowledge of acidic functional groups!
