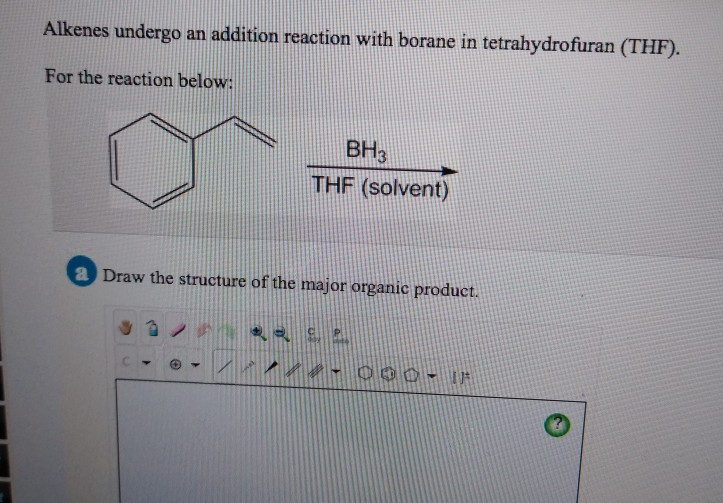
Alkenes Undergo An Addition Reaction With Borane In Tetrahydrofuran Thf

Okay, gather ‘round, everyone. Let’s talk about something that sounds super fancy, but is actually kind of… well, let’s just say it’s a bit of an acquired taste. We’re diving into the world of organic chemistry, a place where molecules do the darndest things. And today, we’re focusing on a particular dance they do.
Imagine a bunch of party animals, right? They’re all excited, ready to have a good time, and they’re looking for new experiences. That’s sort of like our alkenes. They’ve got these double bonds, which are like their invitations to a party, always ready to mingle.
And who’s the VIP guest at this particular party? It’s a character called borane. Now, borane might sound a bit intimidating, like a stern librarian, but it’s actually quite the social butterfly, in its own peculiar way. It’s got this eagerness to join in.
The setting for this whole shindig? It’s a cozy little place called tetrahydrofuran. You can just call it THF. Think of THF as the super chill, laid-back venue. It’s the kind of place where everyone feels comfortable, and things can get a little… interesting.
So, we have our enthusiastic alkenes, our eager-to-please borane, and our chill THF. What happens when they all get together? Well, they engage in what us chemists call an addition reaction. Sounds dramatic, doesn’t it? Like a superhero movie plot.
But really, it’s more like a gentle merging. The double bond of the alkene, that party invitation, gets to meet the borane. And they decide to… well, to add to each other. It’s not a violent breakup or anything. It’s a polite joining.
Think of it like this: the alkene is a bit like a handshake. It’s open to new connections. The borane is extending its hand, ready to grasp. And THF is just there, providing the good vibes, making sure everything goes smoothly.
Now, here’s where it gets a little quirky. This addition reaction isn't just a free-for-all. There’s a bit of a rule to it. The borane, in its eagerness, tends to attach itself to the less crowded side of the alkene’s double bond. It’s like the shyest guest at a party, heading for the corner table.
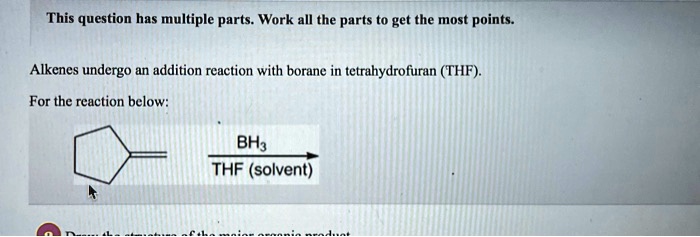
We chemists have a fancy term for this preference: anti-Markovnikov addition. I know, I know, sounds like a mouthful. But essentially, it means the borane plays by slightly different rules than some other reactants. It’s the rebel of the addition reaction world, in a very understated way.
It’s almost as if the borane thinks, “You know what? I’m going to go where the action isn’t as intense, and make my presence known there.” It’s a subtle form of rebellion, really. Not loud, just… different.
And THF, our faithful host, is crucial. It’s not just sitting there looking pretty. THF actually helps stabilize the borane. Borane can be a bit unstable on its own, like a jittery performer before a show. THF gives it a steadying presence, a friendly pat on the back.
So, the alkene offers its double bond, a place to connect. Borane, with its ready willingness, steps in. And THF provides the calm atmosphere that allows this whole thing to happen without a fuss. It’s a three-way partnership, really.
The result of this little get-together? You get a new molecule. The double bond is gone, replaced by single bonds where the borane has made its connection. It’s like the party’s over, and everyone’s gone home, but they’ve left a lasting impression.
And the best part? This reaction is incredibly useful! It’s not just some abstract chemical curiosity. It’s a building block for creating more complex molecules. It’s how we start to assemble the ingredients for… well, for a lot of things you encounter every day.

Think of it as a molecular construction project. The alkene is the raw material, borane is a specific type of tool, and THF is the workspace. You use this process to shape and build. It’s fundamental to making all sorts of organic compounds.
Now, I know what you might be thinking. “An addition reaction with borane in THF? Really?” And I get it. It sounds like something your chemistry teacher said at 8 AM on a Monday. But there’s a certain elegance to it, isn't there?
It’s this quiet efficiency. Borane doesn’t make a huge fuss. It just steps in and does its job. It’s the unsung hero of many organic synthesis pathways. Always there, doing its thing.
And the fact that it prefers the less hindered side? It adds a layer of predictability. We can, to a certain extent, control where these new connections form. It’s like knowing your friend will always pick the window seat.
It’s these seemingly small, specific reactions that underpin so much of our modern world. From medicines to materials, these fundamental chemical dances are happening all the time. And the alkene-borane-THF trio is a quiet but significant player.
So, next time you hear about alkenes, borane, THF, and addition reactions, don’t groan. Smile. Because you know it’s just a bunch of molecules having a surprisingly useful and orderly party. It’s chemistry, but with a bit of a wink.
It’s not the most glamorous reaction, I’ll grant you that. It doesn’t have the explosive excitement of some others. But it’s reliable. It’s consistent. It’s the dependable friend who always shows up.
And sometimes, in the grand scheme of chemical transformations, that’s exactly what you need. A steady hand, a predictable outcome, a friendly solvent. A little bit of anti-Markovnikov flair, courtesy of borane.
So yes, alkenes undergo an addition reaction with borane in tetrahydrofuran (THF). And while it might not be the talk of the town, it’s a conversation worth having, if only for its quiet importance. It’s a testament to the fact that even the most ordinary-sounding chemical processes can be quite remarkable.
It’s chemistry that gets things done, without needing a spotlight. It just happens, and then, poof, you have a new molecule. A silent, efficient transformation. And that, my friends, is pretty neat.
It’s like the chemistry equivalent of a perfectly brewed cup of tea: simple ingredients, but a delightful and essential outcome.
The alkene, with its double bonds, is like the open invitation. The borane is the guest who’s always happy to join. And THF? THF is the perfect host, ensuring everyone feels comfortable and the conversation flows smoothly.
It’s this blend of structure and spontaneity that makes organic chemistry so fascinating. You have these predictable patterns, like the tendency for borane to stick to the less crowded side, which we call anti-Markovnikov addition. It’s a rule that helps us guide the reaction.
But within that predictability, there’s a certain freedom. The alkene can react with a variety of borane compounds, and THF is always ready to facilitate. It’s a collaborative effort.
And let’s not underestimate the role of THF. It’s not just a bystander; it’s an active participant in making the borane more manageable. Think of it as the friendly mediator, preventing any awkward chemical disagreements.
So, while the name might sound a bit technical, the process itself is a beautiful illustration of how molecules interact. It’s about building and transforming, one controlled addition at a time.
It’s the kind of reaction that might not win any awards for flashiness, but it’s a solid performer. It’s the reliable workhorse of organic synthesis, quietly contributing to the creation of complex molecules.
And perhaps that’s my unpopular opinion: these seemingly mundane reactions are actually quite brilliant. They’re the unsung heroes that allow us to create the world around us.
So, let’s raise a (metaphorical) beaker to the alkene, the borane, and the ever-so-helpful THF. They’re doing important work, one addition reaction at a time.
