Alcohols Ethers And Phenols Contain Oxygen With Only Single Bonds
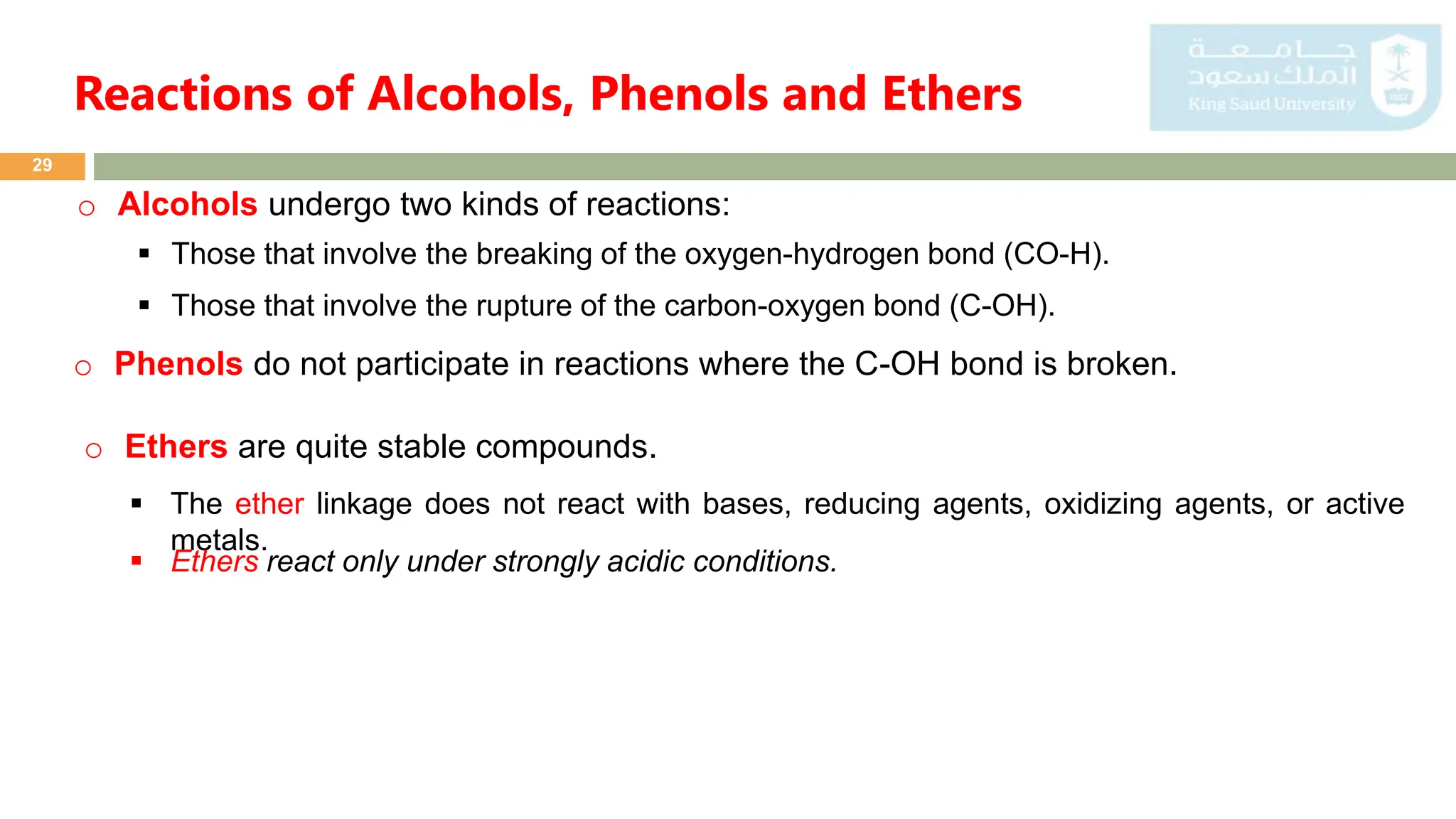
Get ready to dive into the wonderfully wacky world of oxygen-containing molecules that are as common as your morning coffee, but maybe a bit more mysterious! We're talking about a special crew: alcohols, ethers, and phenols. Now, don't let the fancy names scare you. These guys are basically the friendly neighborhood oxygen atoms, hanging out with their carbon buddies, and they're doing it all with just the simplest, most elegant single bonds. Think of it like a gentle handshake, not a super-tight hug, when they connect!
Let's start with the rockstars of this group, the alcohols. You’ve definitely met these guys! The most famous one? Ethanol! Yep, that’s the stuff in your celebratory bubbly or the soothing sip after a long day. It’s also the secret ingredient that makes hand sanitizers so darn effective at zapping those pesky germs. See? Practical and fun! Alcohols are like the life of the party, always bringing something useful to the table. They've got a hydroxyl group (that’s just a fancy term for an oxygen atom attached to a hydrogen atom, like a little dangling accessory) glued to a carbon chain. This little OH group is a real multitasker. It makes alcohols behave in all sorts of cool ways, from dissolving things like a charm to being a crucial part of those yummy flavors in fruits.
Imagine a tiny little water molecule that’s decided to get a bit more adventurous. It’s swapped one of its hydrogen atoms for a carbon chain. Voila! You’ve got yourself an alcohol. It’s so simple, so pure, so… single-bonded! No complex double bonds here, just a steady, reliable connection. This simplicity is what makes them so versatile. They can be super small and nimble, like methanol (which, by the way, is not for drinking – it’s much more serious business, like fuel!), or they can be long and elegant, like the fatty alcohols that make your lotions feel so smooth and luxurious. They’re the Swiss Army knives of organic chemistry, always ready to lend a hand (or an oxygen atom).
Next up, let’s meet the ethers. If alcohols are the rockstars, ethers are the cool, laid-back friends who are always there for you. They’re basically like two alcohol molecules that have gotten together and, through a little chemical magic, decided to ditch their hydrogen atoms and form a bridge. So, instead of an oxygen atom holding hands with just one carbon chain, it’s holding hands with two! Think of it like a little oxygen swing set, connecting two carbon chains. The most common ether you might encounter is diethyl ether, which used to be a go-to for making people fall asleep during medical procedures. Pretty dramatic, right? But today, you’ll find ethers being used as fantastic solvents, great at dissolving all sorts of things without getting too involved themselves. They’re the invisible helpers, the backstage crew that makes the whole show run smoothly.
Ethers are like the perfect roommates. They're stable, they don't cause a fuss, and they're excellent at keeping things in order. Their oxygen atom, with its two trusty single bonds, acts as a reliable connector. They don't have that reactive OH group that alcohols boast, making them a bit more chill. This means they’re less likely to, you know, spontaneously combust (though it’s always wise to be careful!). They’re also great at carrying other molecules around without interacting too much, which is why they’re so prized in laboratories for carefully controlled reactions. They're the unsung heroes, the reliable workhorses that we often overlook but are absolutely essential.
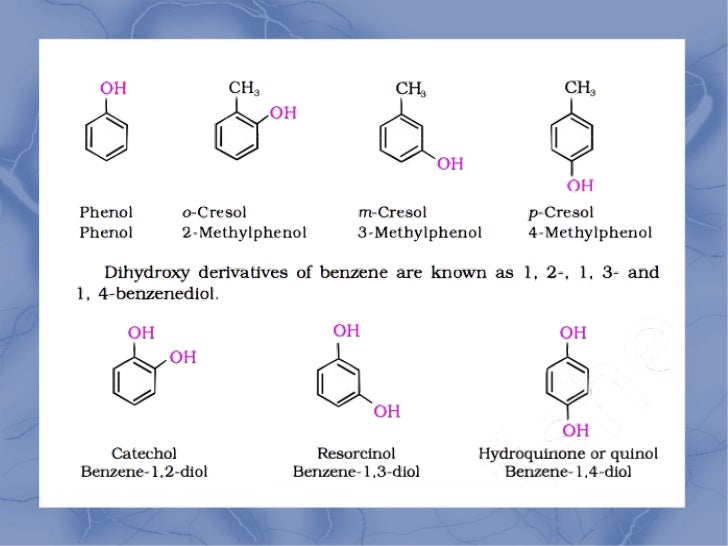
And finally, let’s introduce the glamorous ones, the phenols. These guys are like the fancy cousins of the alcohols, with a special twist. They’re like alcohols that have decided to attach their hydroxyl group (that ever-present oxygen and hydrogen) directly to a special kind of carbon ring called a benzene ring. Imagine a perfectly structured hexagonal arrangement of carbon atoms, and right there, smack dab in the middle of the party, is our little OH group. It’s a bit like a crown jewel on a very sophisticated necklace!

Phenols have a bit of an attitude, and that’s part of their charm. Because they’re attached to that stable benzene ring, they act a little differently than regular alcohols. They can be surprisingly acidic, meaning they’re not afraid to let go of their hydrogen atom if the situation calls for it. This makes them super useful as disinfectants. Think of phenol itself, the original antiseptic – it was a real game-changer for hygiene! They're also found in nature, contributing to the wonderful aromas of things like vanilla (that’s vanillin, a phenol!) and giving that slightly bitter taste to things like tea. They’re the sophisticated intellectuals of the oxygen-containing world, with a bit of a punch.
The key takeaway here, the really cool secret that unites these three amazing groups – alcohols, ethers, and phenols – is their use of simple, beautiful, single bonds involving oxygen. No drama, no complicated structures, just pure, unadulterated connectivity. This elegant simplicity is what allows them to be so diverse and so vital in our everyday lives, from the medicines we take to the foods we eat and the materials we use. They’re the quiet achievers, the dependable friends, and the elegant performers, all powered by the humble, yet mighty, single bond of oxygen!
