Accurately Measure Approximately Within 10 1.00 G Of Sodium Hydroxide

Alright, gather 'round, you magnificent chemists-in-training and kitchen wizards! Today, we're diving headfirst into the thrilling, the electrifying, the downright spectacular world of getting your measurements just right. We're talking about coaxing those tiny, powerful crystals of sodium hydroxide into submission, aiming for a target that's so close, it's practically whispering sweet nothings to our measuring device. We're going for that sweet spot: within a teeny-tiny, microscopic, barely-there 1.00 gram of our desired amount. Yeah, you heard me. We're not aiming for "kinda close" or "good enough for government work." We're aiming for precision that would make a Swiss watchmaker weep with joy!
Now, I know what you're thinking. "1.00 gram? That sounds like a lot! Or is it a little? What even is a gram when it comes to these things?" Fear not, my friends! Think of it this way: imagine you're trying to hit a bulls-eye on a dartboard. You don't want to be a foot away, do you? No, you want to be so close that the dartboard knows you were there. You want to be within that glorious, triumphant circle. That 1.00 gram? That's our super-duper, extra-special, VIP bulls-eye zone!
Let's paint a picture, shall we? Picture yourself baking the most epic batch of cookies known to humankind. You've got the chocolate chips practically singing opera, the butter is at that perfect, creamy stage, and then... the flour. You're scooping and leveling, and your goal is, say, 300 grams of flour. If you're off by a whopping 50 grams, those cookies might turn out flatter than a pancake that's been run over by a steamroller. But if you're off by just 1 gram? Pfft! Your cookies will be legendary. They'll have the perfect chew, the ideal crisp, the kind of texture that makes people question their life choices because they can't believe something so delicious exists. That's the magic of being close!
And that's precisely what we're aiming for with our magnificent sodium hydroxide. This stuff, it's a powerhouse. It's the secret ingredient in so many amazing things, from making super-slippery soap to, well, let's just say it's involved in some truly fascinating transformations. And when you're working with such a potent player, you want to be its partner, not its boss who's wildly guessing. You want to be on the same page, speaking the same granular language.
So, how do we achieve this seemingly impossible feat of pinpoint accuracy? It's not about having a secret stash of alien measuring tools or chanting ancient incantations (though I wouldn't judge if you did!). It's about employing some tried-and-true, common-sense brilliance. First off, you need a scale. Not just any scale, mind you. We're talking about a scale that's got some oomph. We need a digital scale that can measure down to at least the tenths of a gram, if not the hundredths. Think of it as a super-spy for tiny weights. It's going to be your trusty sidekick in this grand adventure.
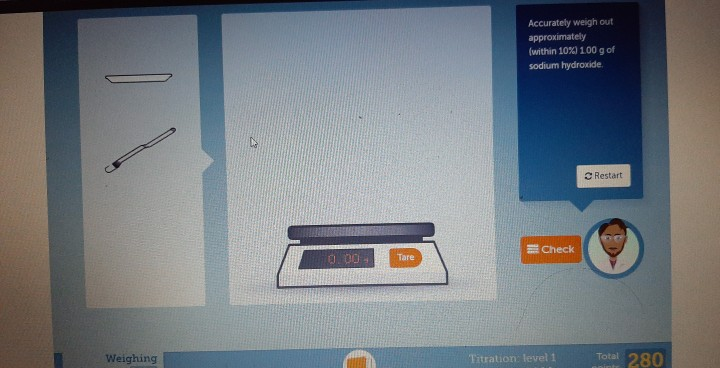
Now, when you're getting ready to weigh out your precious sodium hydroxide, remember this golden rule: tare. What's taring? It's like giving your scale a fresh start. You put your container on the scale, press that magical "tare" button, and poof! the scale reads zero. This means it's only going to measure what you add after that, ignoring the weight of the container itself. It's like telling your scale, "Okay, we're starting from scratch, buddy. Let's get serious!"
Imagine you're adding sugar to your coffee. You want exactly two spoonfuls, not two spoonfuls plus a little bit of spoon. Taring is the science equivalent of that!
3 Ways to Measure Grams - wikiHow
Next up, the art of the slow pour. This is where patience becomes your greatest virtue. You've got your sodium hydroxide ready to go. You're holding your scoop or spatula, and you're approaching the scale with the reverence of a knight approaching a dragon. You start by adding a bit more than you think you need. Then, with the delicacy of a butterfly landing on a dewdrop, you start slowly taking tiny bits away. Or, if you're a bit under, you add minuscule, almost imperceptible pinches. It's a dance, a gentle coaxing. You're not shoveling; you're delicately nudging.
And don't forget about static electricity! Sometimes, those little crystals can get a bit clingy. If you're working in a super dry environment, you might find your readings doing a little jig. A humidifier can be your friend, or even just a gentle tap on the container to help settle things. It's all part of the fun, the quirky charm of the laboratory!
The key takeaway here, my friends, is that achieving that within 1.00 gram goal for sodium hydroxide isn't about brute force; it's about finesse. It's about respecting the power of the material and using the right tools and techniques to get the job done with precision and a smile. When you nail it, when you see that number on the scale hover and settle exactly where you want it, there's a little spark of triumph that ignites. You've tamed the beast, and you've done it with style. So go forth, measure with gusto, and revel in the glory of being almost perfect, but in the most beautifully accurate way possible!

