A Student Balances The Following Redox Reaction Using Half-reactions.

Ever feel like your life is a constant juggling act? Between lectures, study sessions, social hangouts, and maybe even a part-time gig, it's easy to feel like you're on the verge of a beautifully chaotic explosion. Well, let us introduce you to the world of redox reactions, specifically, how a clever student named Alex uses them as an unlikely metaphor for mastering their own hectic schedule.
Alex, a bright spark in their university's chemistry program, has discovered that the seemingly daunting task of balancing complex chemical equations has a surprising kinship with the everyday art of time management. And no, this isn't your grandpa's dusty textbook lecture. We're talking about a chill, modern take on science that’s as relevant to your coffee-fueled mornings as it is to the Big Bang.
So, what exactly is a redox reaction? Think of it as a chemical dance of electrons. Some atoms are giving away electrons, and others are taking them. This transfer is what drives the reaction. Alex's secret? Breaking down these complex reactions into simpler, manageable steps, known as half-reactions. It’s all about identifying who's losing electrons (oxidation) and who's gaining them (reduction). Sound complicated? Stick with us; it’s less "Mad Men" boardroom and more "Friends" coffee shop chat.
The Oxidation Tango
In Alex’s world, the "oxidation half-reaction" is like those moments when you commit to something. You’re giving something up, sacrificing a bit of your time or energy. For instance, choosing to go to that early morning seminar instead of hitting snooze. That’s your electrons (your precious sleep!) being given away. You’re oxidizing your relaxation for the sake of knowledge. Go you!
Alex uses a classic example: the reaction between zinc metal (Zn) and copper ions (Cu²⁺). In the oxidation half-reaction, zinc metal loses two electrons to become a zinc ion: Zn → Zn²⁺ + 2e⁻. It's a straightforward transaction, much like deciding to pay for that artisanal coffee when your budget is tight. You're losing money (electrons), but gaining that delicious caffeine boost (a positive outcome, hopefully!).
Practical Tip: Identify your "electron donors" in life. What are you readily giving up? Is it screen time for exercise? Social commitments for quiet study? Recognizing these “sacrifices” is the first step to understanding your personal energy flow.
The Reduction Rumba
Now, for the "reduction half-reaction." This is where you receive those electrons. It’s the payoff, the reward, the moment something is gained. In our chemical example, the copper ions (Cu²⁺) are eager to snatch up those electrons that the zinc so generously offered: Cu²⁺ + 2e⁻ → Cu. The copper ions are being reduced because they're gaining electrons.

Think about it like this: you’ve just aced a tough exam. All those late-night study sessions (your oxidation) have paid off. You're receiving that excellent grade, that sense of accomplishment. Your brain is essentially gaining those "knowledge electrons." It’s the beautiful symmetry of action and consequence, just like in a good chemical reaction or a well-earned nap after a productive day.
Alex’s favorite analogy? The transfer of energy in a well-organized playlist. When Alex meticulously curates a study playlist, they're "oxidizing" their time by choosing tracks. When they actually listen and feel the vibe, their focus and mood are "reduced" or enhanced by the music. It’s a win-win, a perfect balance of effort and reward.
Fun Fact: The terms "oxidation" and "reduction" originally referred to reactions involving oxygen. Oxygen is a highly electronegative element, meaning it loves to grab electrons. So, when something reacted with oxygen, it was thought to be "oxidized." The opposite, gaining electrons, was then logically dubbed "reduction." Science history, it’s wild!
Putting It All Together: The Full Equation Shuffle
Balancing a redox reaction is all about making sure the number of electrons lost in the oxidation half-reaction exactly equals the number of electrons gained in the reduction half-reaction. If you’ve got 2 electrons being donated, you need exactly 2 electrons being accepted. No more, no less. It’s like ensuring your budget perfectly accounts for your income and expenses – no black holes where electrons mysteriously disappear!
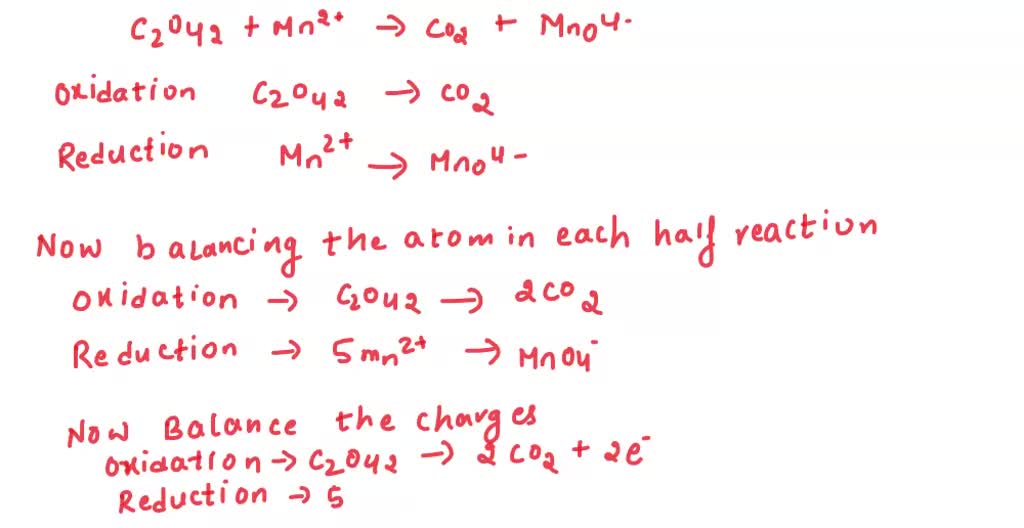
Alex applies this to their schedule. They first identify their "electron donors" – the activities that cost them energy or time (like intense study or a demanding extracurricular). Then, they identify their "electron acceptors" – the things that give them back energy or are the goals of their efforts (like a good grade, a fun social event, or simply feeling rested). The key is to make sure the "energy gained" perfectly balances the "energy spent."
For the Zn and Cu²⁺ example, the balanced overall reaction is: Zn + Cu²⁺ → Zn²⁺ + Cu. See? The two electrons transferred are accounted for on both sides. It’s neat, it’s tidy, it’s scientifically satisfying.
Cultural Reference: Think of it like a perfectly executed dance routine. Every move (electron transfer) has a counterpart, a lead and a follow, ensuring the whole performance is in sync. Whether it's a hip-hop battle or a ballroom waltz, balance is key to looking good and feeling right.
Navigating Different Environments: Acidic vs. Basic Media
Here’s where it gets a little more nuanced, much like navigating the social scene on campus. Redox reactions can occur in different "media" – acidic or basic solutions. And just like certain social situations require a different approach, balancing equations in these media involves different techniques.
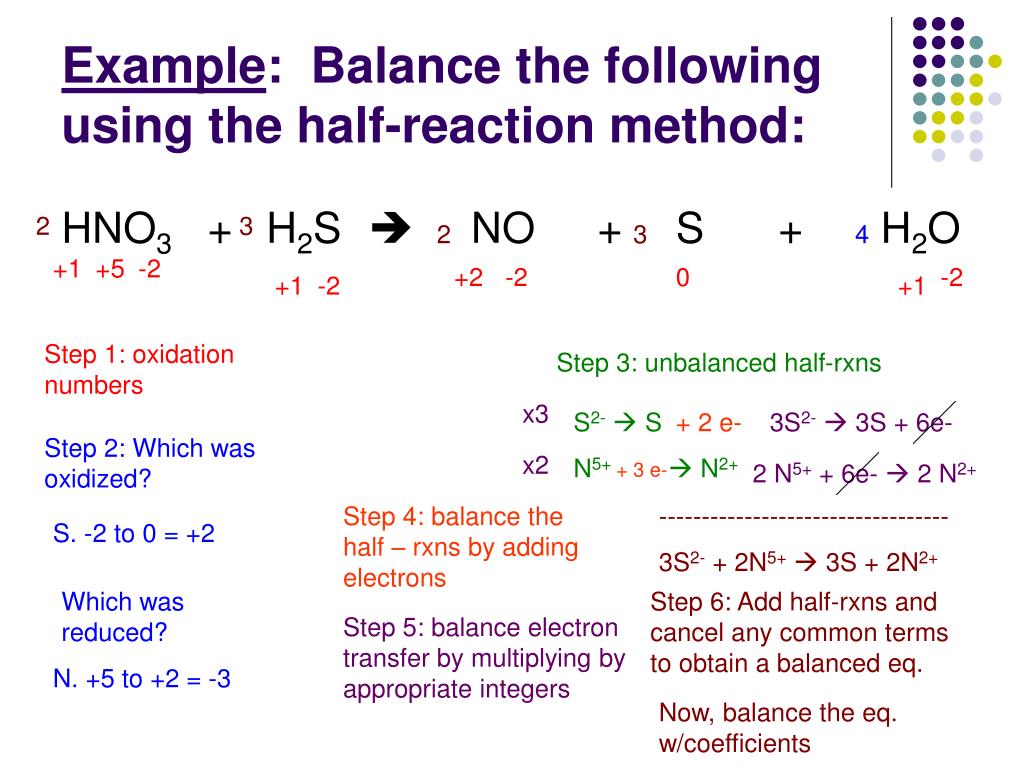
In acidic solutions, you can add hydrogen ions (H⁺) and water molecules (H₂O) to balance things out. It’s like having a toolkit of readily available resources. Alex sees this as their "during-exam week" strategy: piling on the coffee (H⁺) and staying up late with friends for a quick, energizing chat (H₂O). It’s about using available tools to keep the reaction (their brain) going.
In basic solutions, things get a bit trickier. You add hydroxide ions (OH⁻) and water. It requires a bit more finesse, a subtler touch. Alex likens this to their "weekend recovery" mode. Instead of blasting through tasks, they’re gently rehydrating with water (OH⁻) and taking moments of calm reflection (H₂O). It’s about a more restorative, less aggressive approach.
Practical Tip: Recognize your "media." Are you in an "acidic" phase of your life where you need to aggressively push forward, or a "basic" phase where gentle self-care is more appropriate? Adjust your strategies accordingly.
The Art of Balancing It All
Alex's journey into redox balancing isn't just about passing chemistry; it's about a fundamental shift in perspective. They've learned that complex challenges can be demystified by breaking them down into their core components, understanding the individual transfers, and ensuring everything adds up in the end.

This applies to everything. When Alex feels overwhelmed by multiple deadlines, they don't just stare at the mountain of work. They break it down: what's the most urgent task (the thing that needs electrons most urgently)? What resources do they have available (H⁺ and H₂O)? Who can help them (other students, TAs)? They identify the "electron donors" (time, effort) and "electron acceptors" (completed assignments, good grades) for each task, and then meticulously balance their time to ensure a positive outcome.
It’s about more than just stoichiometry; it’s about self-awareness. It’s about understanding your own energy cycles, your commitments, and your rewards. It’s about seeing your life as a dynamic chemical system, constantly undergoing transformations, and learning to guide those transformations for optimal results.
Fun Fact: Did you know that the human body is a complex network of redox reactions? From the energy production in your cells to the way your muscles contract, electrons are constantly being transferred. You're basically a walking, talking redox machine!
A Little Reflection
So, the next time you're feeling swamped, or when life throws you a curveball that feels as complicated as a multi-step redox reaction in basic solution, take a deep breath. Remember Alex. Remember the half-reactions. Identify what you’re giving, what you’re gaining, and how you can ensure the balance is in your favor. It might just be the key to transforming that chaotic explosion into a beautifully controlled, productive, and even enjoyable, chemical process called life.
