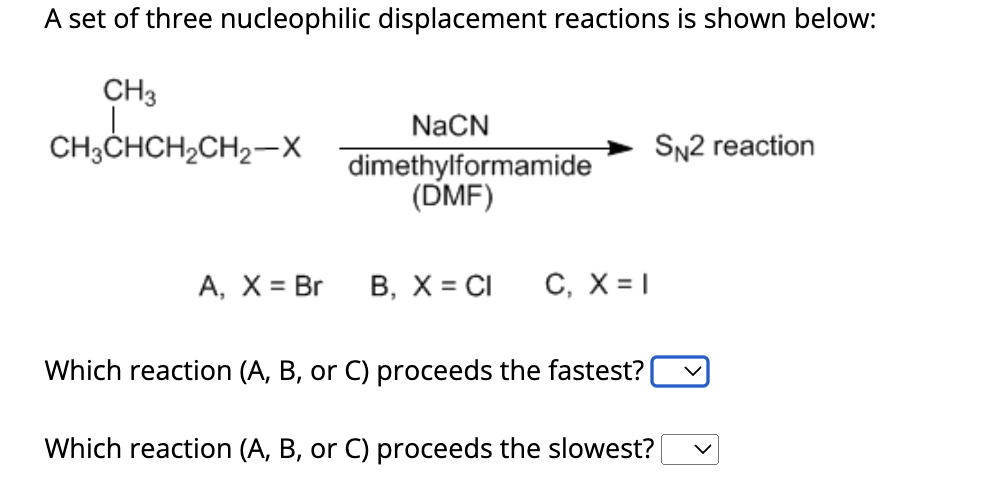
A Set Of Three Nucleophilic Displacement Reactions Is Shown Below
Hey there, science fans and curious cats! Ever wondered what goes on behind the scenes of, like, everything? It’s all about tiny little dance parties, and today we’re crashing one of the coolest: nucleophilic displacement reactions. Sounds fancy, right? But trust me, it’s way more fun than it looks. Think of it like a microscopic game of musical chairs, but with atoms and molecules. And we’ve got a sweet little trio of these reactions to peek at!
So, what’s the big deal? Well, these little shindigs are the backbone of so much. They’re how we make medicines, create cool new materials, and even how our bodies do their amazing work. It’s basically chemistry in action, and it’s a blast to understand how it all clicks together. We’re going to dive into three specific examples, and I promise, no dry textbook stuff here. Just pure, unadulterated chemical fun!
The Players: Who’s Who in the Reaction Game?
Before we jump into the action, let’s meet our star players. We’ve got two main characters in this drama: the substrate and the nucleophile. The substrate is like the dance floor – it’s the molecule that’s got something to lose. And the nucleophile? That’s our eager dancer, ready to swoop in and take its place. The word "nucleophile" itself is kinda neat, isn't it? It means "nucleus-loving." These guys are positively charged or have a negative charge, or just a spare electron pair, and they’re always attracted to the positive bits in other molecules. They’re basically the romantic heroes of the chemical world, always looking for a sweet spot to land.
And then there’s the leaving group. This is the bit of the substrate that gets kicked to the curb. It’s like the old dance partner who’s gotta make way for the new. Sometimes they leave gracefully, sometimes not so much. But without a good leaving group, the whole dance can’t happen. It’s all about finding the right molecule with a good "leaving" personality!
Reaction 1: The Speedy Swinger – SN2 Style!
Alright, let’s get to our first act. This is the SN2 reaction. The "SN" stands for nucleophilic substitution, and the "2" means two molecules are involved in the slow, rate-determining step. This is like a super-efficient, one-step tango. The nucleophile comes in from the back, giving the leaving group a little nudge, and bam! they switch places. It’s a coordinated effort, all happening at once.
Imagine our nucleophile as a super-fast delivery driver. It sees a package (the substrate) with an address label (the leaving group) that’s about to expire. The driver zooms in, grabs the package, and in the same motion, swaps it with the new address label. No waiting! This happens in one fell swoop. Super efficient, right?
A quirky fact? SN2 reactions often lead to an inversion of stereochemistry. Think of it like a glove turning inside out. The configuration at the carbon atom flips. It’s a little like the nucleophile giving the leaving group a literal shove from the opposite side, causing everything to flip around. Pretty neat, huh? It’s a testament to how precise these molecular rearrangements are.
Why is this fun to talk about? Because it’s a direct, no-nonsense interaction. It’s the chemical equivalent of a quick, decisive handshake. Plus, the idea of a molecule flipping itself inside out is just delightfully weird. It’s a tiny cosmic ballet of atoms.
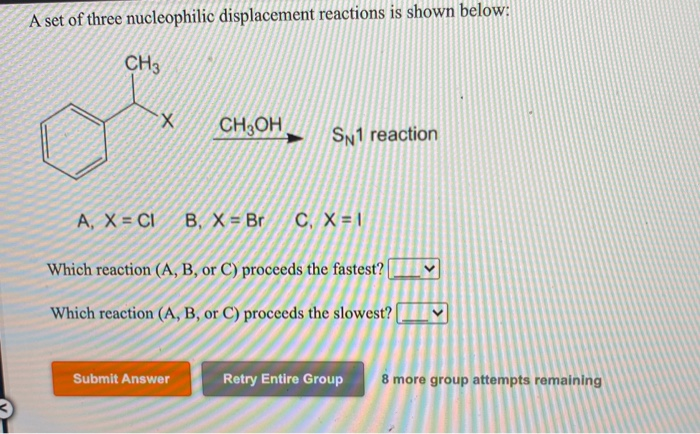
Reaction 2: The Patient Planner – SN1 Style!
Now, for our second act, we have the SN1 reaction. The "1" here means only one molecule is involved in the slow, rate-determining step. This is more of a slow-burn romance. It’s a two-step process.
First, the leaving group says "peace out!" and leaves the substrate all by itself. This creates a little positively charged dude called a carbocation. Think of it as a lone ranger, feeling a bit vulnerable. Then, the nucleophile, seeing this opportunity, swoops in and forms a new bond. It's like the leaving group leaves, and then the new guy arrives.
This is less of a direct collision and more of a strategic move. The substrate has to first break apart, creating an intermediate. This carbocation intermediate is pretty unstable, kind of like a person standing alone in a room full of strangers. It's really looking for some company, and fast! The nucleophile sees this and is all like, "Hey, need a friend?"
Why is this fun? Because it introduces a whole new character: the carbocation! These intermediates are super important in organic chemistry. They’re like fleeting celebrities, existing for a very short time but having a huge impact. Also, SN1 reactions can sometimes lead to a mix of configurations (a racemic mixture) because the nucleophile can attack from either side of the planar carbocation. It’s less about perfect inversion and more about a more relaxed, "come as you are" attitude.
The pace is different, the mechanism is different, and the outcome can be different. It’s like comparing a sprint to a marathon. Both get you there, but the journey is totally unique. This is where the real nuance in chemistry starts to shine.
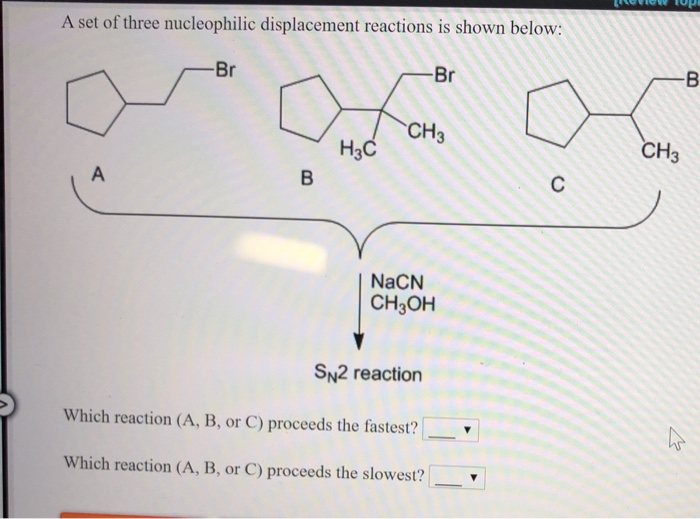
Reaction 3: The Competitive Couple – Competition and Factors
So, we’ve seen SN2 is fast and SN1 is… well, more drawn out. But what determines which one happens? Ah, this is where it gets really interesting! It’s not just about the molecules themselves, but also the conditions. We’re talking about the solvent (what the reaction is dissolved in) and the structure of the substrate.
Think of it like a dating app for molecules. SN2 reactions are like the ones that prefer a crowded, bustling club – they like polar aprotic solvents (solvents that are polar but don’t have a "sticky" hydrogen atom). These solvents help the nucleophile be more aggressive. SN1 reactions, on the other hand, prefer a more chill, cozy atmosphere – they like polar protic solvents (solvents with "sticky" hydrogens, like water or alcohol). These solvents help stabilize the carbocation intermediate by surrounding it.
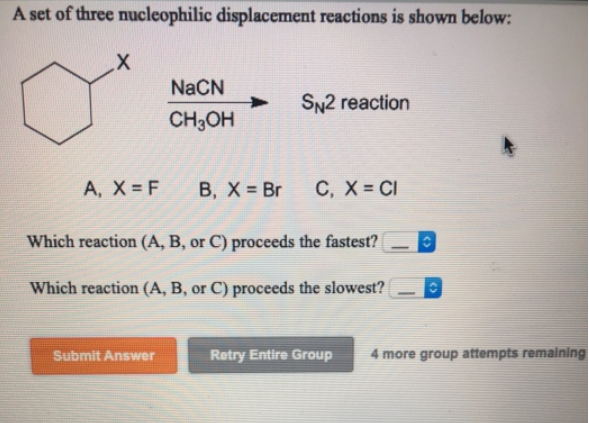
And the substrate structure? For SN2, it's like a crowded dance floor. If the carbon atom where the reaction happens is too bulky (like in tertiary alcohols), the nucleophile has a hard time getting in. So, SN2 favors methyl and primary substrates. For SN1, the carbocation is the key. More stable carbocations form more easily. Tertiary carbocations are super stable, so SN1 favors tertiary substrates. It’s all about who’s got the most room to maneuver or who can best handle being alone for a bit!
Why is this fun? Because it shows that chemistry isn't just a set of rigid rules. It's a dynamic interplay of forces. You can tweak the conditions and totally change the outcome. It’s like being a molecular matchmaker, orchestrating the perfect reaction. You can push it towards a speedy SN2 or encourage a more leisurely SN1. The power is in your hands (or in the beaker, rather!).
The Grand Finale: Why It All Matters
So there you have it! Three fundamental ways molecules swap partners. From the direct dive of SN2 to the stepwise shuffle of SN1, and the factors that decide who leads. These reactions might seem small and abstract, but they're the building blocks of so much in our world. They’re how scientists create new drugs to fight diseases, how we develop advanced materials for our gadgets, and even how our bodies function at the most basic level.
Understanding these little chemical dances helps us unlock the secrets of life and build a better future. It’s a reminder that even the tiniest interactions can have huge consequences. So next time you’re looking at a chemical formula, don’t just see letters and numbers. See a dance floor, eager dancers, and exciting transformations waiting to happen. It’s a whole universe of fun, just waiting to be explored!
