A Bronsted Lowry Base Is Defined As A Substance That

Alright, let’s dive into the quirky world of chemistry, shall we? Specifically, we’re going to chat about something called a Bronsted-Lowry base. Now, don't let the fancy name scare you. Think of it like this: life is full of things that just want to take. And in the chemical world, a Bronsted-Lowry base is precisely one of those things.
Imagine you’re at a potluck. Everyone’s brought their amazing dishes. But there’s always that one person, right? The one who seems to gravitate towards the best snacks first. They don't necessarily make the snacks, they just… well, they accept them. They’re the ultimate snack connoisseur, the VIP of the appetizer table. A Bronsted-Lowry base is kind of like that person, but instead of mini quiches, they’re all about protons.
Yes, protons. Those tiny, positively charged particles hanging out in the nucleus of an atom. In the grand scheme of chemical reactions, protons are pretty darn important. They're like the VIP guests at a chemical party. And a Bronsted-Lowry base? It's the eager host who's ready to receive these important guests. They’re the ultimate proton acceptors. They’re not sending protons out into the world willy-nilly. Oh no. They’re waiting, with open arms (or, you know, open electron pairs), to snag a proton that's being offered up.
Think about it this way. You have a friend who’s always borrowing your favorite pen. They don’t have one themselves, but when you offer, they’re more than happy to take it. That’s your friend being a "pen acceptor." A Bronsted-Lowry base is the chemical equivalent of that friend, but for protons. They're the substance that, when it sees a proton floating around, goes, "Ooh, yes please! I'll take that!"
This is where the "Bronsted-Lowry" part comes in. These two brilliant minds, Johannes Bronsted and Thomas Lowry, had this brilliant idea. They looked at acids and bases and thought, "What if we define them by what they do with protons?" Acids, they said, are proton donors. They’re the generous folks at the potluck who are handing out their homemade cookies. And bases? Well, bases are the ones who are ready and willing to catch those cookies. They are the proton acceptors.

It's a rather elegant, dare I say, simple definition. It doesn’t get bogged down in all sorts of complicated theories about hydroxide ions and things. It’s all about the proton handshake. An acid gives a proton, and a base receives it. Easy peasy, lemon squeezy. Or, perhaps, easy peasy, proton-y cheesy?
So, if you see a chemical reaction where one substance is readily snatching up a proton from another, you can bet your bottom dollar (or your spare proton) that you're looking at a Bronsted-Lowry base in action.
Acids and Bases. - ppt download
It’s this idea of transfer. Everything’s a transfer in chemistry. Energy transfers, electron transfers, and yes, proton transfers. And the Bronsted-Lowry theory just highlights this fundamental exchange. It’s like a chemical game of tag, where the proton is "it," and the base is the one who's always tagging it, metaphorically speaking.
Now, I’ve got a bit of an unpopular opinion here. While other definitions of bases exist, and they’re perfectly valid in their own right, there’s something so… satisfyingly direct about the Bronsted-Lowry definition. It’s like getting straight to the point. No fluff, no unnecessary jargon. Just a clear picture of what’s happening.
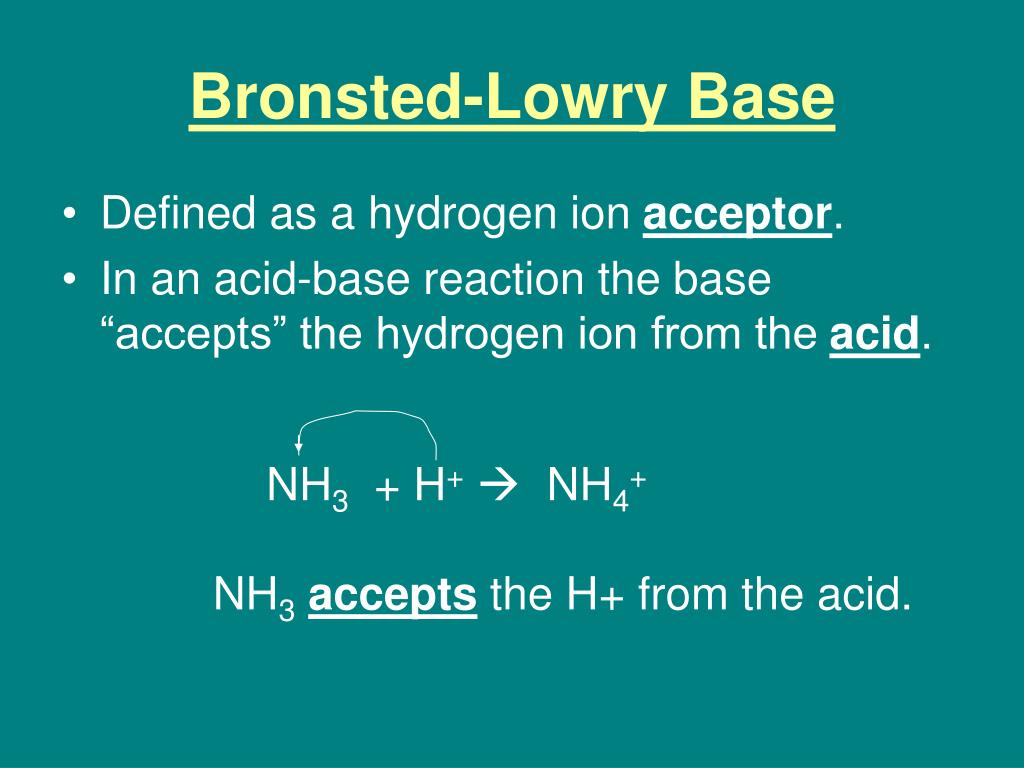
Think of it in everyday terms. You know how some people are just natural listeners? They’re great at taking in what you have to say. They’re the quiet ones who absorb your stories. A Bronsted-Lowry base is the chemical equivalent of that excellent listener. It's not trying to shout over anyone; it's just ready to receive. It's the patient friend who's always there to lend an ear, or in this case, a pair of electrons to bond with a proton.
It’s also kind of funny to think about. We often associate bases with being, well, basic. But in the Bronsted-Lowry world, they’re actually quite accommodating. They’re not aggressive; they’re receptive. They’re the polite guests who wait to be offered something before they take it, but when it is offered, they’re definitely going to accept. It's a subtle but important distinction.
So, the next time you’re pondering the mysteries of chemical reactions, and you see something eagerly accepting a proton, just remember our potluck analogy. That’s your Bronsted-Lowry base, the ultimate proton acceptor, the VIP of the proton party, always ready to welcome a new proton with open arms (and electron pairs). It’s a simple concept, but it’s the backbone of so much of what happens in the world of chemistry. And honestly? It’s kind of brilliant in its simplicity. Who knew chemistry could be so relatable, right?

